Detection of Mycobacterium bovis and Klebsiella pneumoniae at Bali Cattle Slaughterhouse by culture analysis and PCR
on
Jurnal Ilmu dan Kesehatan Hewan, Agustus 2015
Vol 3 No 2: 51-54
Detection of Mycobacterium bovis and Klebsiella pneumoniae at Bali Cattle Slaughterhouse by culture analysis and PCR
Deteksi Mycobacterium bovis dan Klebsiella pnewmoniae pada Rumah Potong Sapi Bali melalui Analisis kultur dan PCR
I Gede Oka Darsana1, I Nyoman Dibia2, Hapsari Mahatmi3*
1Magisterial Program of Veterinary Medicine, Udayana University, Denpasar, Bali 2Animal Disease Investigation Centre, Denpasar, Bali
3Laboratory of Microbiology, Faculty of Veterinary Medicine, Denpasar, Bali *Corresponding author: hmahatmi@yahoo.co.id
ABSTRAK
Penelitian mengenai prevalensi bovine tuberculosis dan Klebsiella pneumoniae pada rumah potong sapi di Bali dilakukan di Rumah Poyong Pesanggrahan dan Mambal dari Bulan Januari sampai dengan Maret 2015. Pengamatan terhadap lesi dilakukan pada organ paruparu dan limfenodus yang selanjutnya diperiksa melalui analisis DNA untuk Mycobacterium bovis dan kultur untuk isolasi Klebsiella pneumoniae. Dari seluruh sampel, tidak ada sampel yang ditemukan lesi yang mengidikasikan menderita tuberculosis di kedua rumah potong hewan. Namun, Klebsiella pneumoniae teramati pada 1/4513 sampel (0,02%) hanya di Rumah Potong Sap Mambal. Penelitian ini merupakan laporan pertama adanya Klebsiella pneumoniae di Bali. Karenanya, dibutuhkan kehati-hatian terhadap Klebsiellosis yang besifat zoonotik ini dan formulasi management kontrolnya.
Kata kunci: sapi Bali, Mycobacterium bovis, Klebsiella pneumoniae, prevalensi
ABSTRACT
A study to determine the presence and prevalence of bovine tuberculosis and Klebsiella pneumoniae at cattle slaughterhouse in Bali was carried out in Pesanggaran and Mambal abattoirs from January to March 2015.The Lungs and lymph nodes were inspected for lesions and then examined through DNA analysis for Mycobacterium bovis and culture for isolation of Klebsiella pneumoniae. Of the samples examined, no one had lesions suggestive of tuberculosis in two abattoirs.However, Klebsiella pneumoniae was observed 1/4513 sample (0.02%) only in Mambal abattoir. This study is the first report of the presence of Klebsiella pneumoniae in Bali. Therefore, a aware of this zoonotic Klebsiellosis and its control management formulation are need.
Keywords : Bali Cattle, Mycobacterium bovis, Klebsiella pneumoniae, prevalence
INTRODUCTION
Bali cattle plays an important role in supplying beef in Indonesia because it has good quality, high fertilization and low fat percentage (Merliana et al., 2014). The Bali cattle is considered to be well adapted to the country’s harsh environmental tropical conditions. Despite it is superior qualities this breed has weakness. The Bali cattle has a susceptible to Jembrana diseases and Malignant Catarrhal Fever. Another disease, such as Bovine tubercolosis (BTB) and Klebsiellosis until recent years there was a
little concern about these infection in bali cattle.
Bovine tuberculosis is caused by Mycobacterium bovis (bovine tubercle bacillus) which is a member of Mycobacterium tuberculosis complex. This disease is a chronic infectious disease and contagious zoonotic disease of domestic animals, wild animals, and humans. Sometimes this disease can be acute and progressive, specially on calves (Poeloengan et al., 2014). And, Klebsiellosis is a disease that caused by Klebsiella pneumoniae bacteria (Rahmawati, 2009). Klebsiella pneumoniaeis
an opportunistic pathogen on human and also animal (Younan et al., 2013).
In Bali, there have been limited studies to determine the prevalence of Bovine tuberculosis and Klebsiellosis. According to Putra et al. (2013), seroprevalence of bovine tuberculosis in bali cattle from Bangli region was 2.22%. Moreover, former report stated that Klebsiellosis event was found on the cattle lung samples that show the sign of pneumonia from cattle slaughterhouse in Gorontalo (Retnowati & Nugroho, 2015).
This study was aimed to determine the prevalence of BTB at cattle slaughterhouse based on polymerase chain reaction (PCR) techniques and Klebsiellosis based on cell culture methods.
MATERIALS AND METHODS
Sample
Samples were collected from Pesanggaran Abattoir, Denpasar and Mambal Abattoir, Badung. The selection of the cattle sampled at each abattoirs was strictly based on the clinical signs, following postmortem observation of typical granulomatous lesions of Bovine tuberculosis in the lungs or the lymph nodes.
Bacterial Culture and Identification
Culture was conducted in category III containment laboratories. Samples were transported in refrigerated condition, and processed within 24h of collection. Klebsiella pneumoniae bacteria were tested by culturing on MacConkey media. Confirmatory testing of presumptive positives was by Gram colouring test, biochemical and sugar test.
Polymerase Chain Reaction (PCR) Method
The tissue samples showing clinical signs of Bovine tuberculosis were collected and transported on ice pack to laboratory. Genomic DNA were extraction using genomic DNA mini kit. DNA were amplified under standard conditions as described Geneaid Product Information. The primers were 5’-CAGGGATCCACCATGTTCTTAGCGGGT TG-3’ (forward) and 5’-TGGCGAATTT
CTTACTGTGCCGGGGG-3’ (reverse) (Nahar et al., 2011). The reaction was performed in a final volume of 25 μL. After an initial denaturation step (at 94°C, for 3 min), 40 amplification cycles were performed as follows: denaturation at 95°C for 15 second, annealing at 63°C for 15 sec, and extension at 72°C for 30 sec. A final extension was performed at 72°C for 15 min. The amplified PCR products were electrophoresed in 1.5% a g ar o s e g e l .
Data Analysis
The obtained data were analyzed descriptive statistically using SPSS Program for Windows.
RESULTS AND DISCUSSION
Results
A total of 4513 Bali cattle from slaughterhouses were examined. Two tissue samples were collected from cattles that show suspected respiratory lesions. The lungs inspection found pathologic changes such as pneumonia. While, the lymph nodes were showed no pathologic changes. Further confirmation using PCR technique, the both samples were negatif for Mycobacterium bovis (Figure 1).
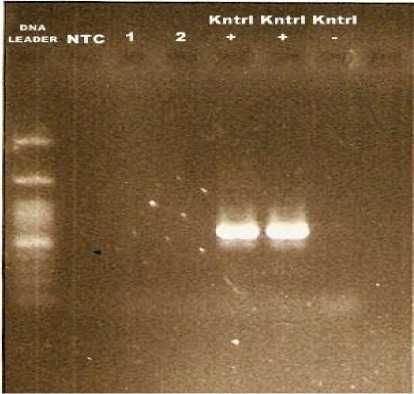
Figure 1. PCR amplification of gene specific for M. bovis . Electrophoresis 1.5% agarose gel. 1,2 the samples, + positive control of M. bovis
Culture analyis demostrated that there was Klebsiella pneumoniae in one sample from two sample that suspected respiratory lesions. The biochemical test and sugar test of the sample were presented in Table 1.
Table 1. The Biochemical and Sugar Test for
Klebsiella pneumonia
|
Biochemical and sugar test |
Result to Sample A |
Klebsiella Pneumonia, Cowan (1974) and Sharma et al., (2014) |
|
Motilitas |
- |
- |
|
Sitrat |
+ |
+ |
|
Gas dari | ||
|
Glukosa |
+ |
+ |
|
Laktosa |
+ |
+ |
|
Sukrosa |
+ |
+ |
|
Methyl | ||
|
Red |
+ |
+ |
|
Voges | ||
|
Proskauer |
- |
- |
|
Indol |
- |
- |
|
Urea |
+ |
+ |
|
Acid/Acid ; |
Acid/Acid ; | |
|
TSIA |
H2S - ; Gas + |
H2S - ; Gas + |
|
Oksidase |
- |
- |
|
Katalase |
+ |
+ |
Discussion
The result of PCR technique in this study confirmed negative for Mycobacterium bovis. Refers to the epidemiology concept that a disease is due to the interaction of agent, host, and environment factors (Martin et al., 1987). The negative result of Mycobacterium bovis as the cause of BTB in this study can be overviewed from those 3 factors. Poeloengan et al. (2014) stated that the susceptible hosts of BTB infection is so wide in range, such as guinea pig, rabbit, mencit, hamster, monkey, horse, dog, cow, pig, cockatoo and fowl. Wild animal that is a permanent reservoir from the Mycobacterium bovis infection is fox (Meles-meles) in Britain, possum (Trichosurus Vulpecula) in New Zealand and monkey in
Indonesia (Poeloengan et al., 2014). BTB agent have the ability to live a couple of days outside of its source (Tarmudji and Supar, 2008), this indicates that in the amount of time the BTB agent still have the ability to infected the host. Mycobacterium bovis infection can spread rapidly towards the cattle especially through the aerosol inhale, from the cough or sneezing of the animal that have tuberkulosis or from the dust particle which contain the agent. The disease also spread rapidly in a very dense location of cattle (Cousins, 2001), or when a wild animal and cattle herd in the same field (Cosivi et al., 1998). While the infection in human, it is usually through the drink of fresh milk and consuming the raw animal product. Besides, the farm worker that keep the animal inside the cage can increase the risk of the infection in aerosol way, from human to animal, or the opposite (Cosivi et al., 1998).
The major finding in the present study is the presence of Klebsiella pneumoniae in cattle. The animal that susceptible to Klebsiellosis infection caused by Klebsiella pneumoniae bacteria are buffalo and cow (Sayed dan Zaitoun, 2009), dog, monkey, guinea pigs, muskrats (Brisse et al., 2006). Horse and camel (Younan et al., 2013). While the red swallow (Milvus milvus), Egypt vulture, Antarctic skua (Catharacta spp.), Red-billed chough (Pyrrhocorax pyrrhocorax), wild turkeys (Cathartes aura), peregrinefalcon (Falco peregrinus) were suspected as the carrier agent of Klebsiella pneumoniae (Sharma et al., 2014).
Factors that may influence the presence of Klebsiella pneumoniae in cattle in Bali are due to environmental factor. Klebsiella pneumoniae can grow under the aerob condition at a temperature of 12-43ºC with the optimal growth at a temperature of 35-37ºC (Rahmawati, 2009). Besides, the environmental factor that causing the Klebsiellosis such as the stress of carriage, the polluted water and environment from the waste of the paper factory and wood finishing textile, waste of the plants product and sugar cane (Brisse et al., 2006). Although the prevalence of Klebsiellosis is small, a proper
postmortem inspection should be practiced effectively at the abattoir, before taking out beef to the public. Besides, the zoonotic agent, Klebsiella pneumoniae, can also cause an economical loss on Bali cattle.
CONCLUSION
The prevalence of Klebsiella pneumoniae in cattle was very low (0.02%). Mycobacterium bovis was not found in cattle slaughterhoused based on PCR technique.
REFERENCE
Brisse S, F Grimont and PAD Grimont. 2006. The Genus Klebsiella. Chapter : 3.3.8, Vol 6: 159–196.
Cosivi O, JM Grange, CJ Daborn, MC Raviglione, T Fujikura, D Cousins, RA Robinson, HFAK Huchzermeyer, IDE Kantor and FK Meslin. 1998. Zoonotic Tuberculosis due to Mycobacterium bovisin Developing Countries. Emerging Infectious Diseases, Vol. 4, No. 1.
Cousins DV. 2001. Mycobacterium bovis infection and controlin domestic livestock. Australian Reference Laboratory for Bovine tuberculosis. Rev. Sci. Tech. Off. int. Epiz.,2001, 20 (1): 71-8.
Merliana MR, IN Wanida and IK Puja. 2014. Polimorfisme Lokus Mikrosatelite BM1329 dan Hubungannya dengan calving interval pada sapi bali. JIKH, 2(2) 117-125.
Martin SW, A Meek and P Willeberg. 1987. Veterinary epidemiology. Iowa : Iowa State University Press.
Nahar Q, Pervin M, Islam M, Khan MAHNA. 2011. Aplication of PCR for the Detection
of Bovine tuberculosis in Catle. J. Bangladesh Agril. Univ. 9(1):73-78.
Poeloengan M, I Komala and SM Noor. 2014. Bahaya Dan Penanganan Tuberculosis. Lokakarya Nasional Penyakit Zoonosis.
Putra PGW, NK Besung and H Mahatmi. 2013. Deteksi Antibodi Mycobacterium
tuberculosa bovis pada Sapi di Wilayah Kabupaten Buleleng, Bangli, dan Karangasem Provinsi Bali (Tesis). Denpasar : Universitas Udayana.
Rahmawati D. 2009. Pengaruh Vaksinasi Kultur Klebsiella Pneumoniae Hasil Inaktivasi Pemanasan Dan Iradiasi Sinar Gamma Terhadap Kondisi Fisik Serta Profil Protein Serum Darah Mencit (Skripsi). Jakarta : Universitas Islam Negeri Syarif Hidayatullah.
Retnowati Y, and TAE Nugroho. 2015.
Pemeriksaan Mikroba Dan Patologi Organ Paru-Paru Sapi Yang Mengalami Pneumoni di Kota Gorontalo. Gorontalo : Universitas Negeri Gorontalo.
Sayed SM and AMA Zaitoun. 2009. Aerobic Bacterial Pathogensof Pneumonic Feedlot Buffalo-Calves, In Assiut Governorate, Egypt. Ass. Univ. Bull. Environ. Res. Vol. 12 No. 1.
Sharma SK, P Sharma and BN Shringi. 2014. Phenotypic and Genotypic Characterization of Klebsiella Pneumoniae Obtained from Egyptian Vultures and Steppes Eagles from India. Israel Journal of Veterinary Medicine.Vol. 69 (3).
Tarmudji and Supar. 2008. Tuberkulosis Pada Sapi, Suatu Penyakit Zoonosis. Wartazoa, Vol. 18 No. 4.
Younan M, IV Glücks, R Podschun and S Bornstein. 2013. Klebsiella Pneumonia: A Commensal And Cause Of Septicaemia In Camel Calves (Camelus Dromedarius). Journal of Camelid Science.
54
Discussion and feedback