Dye Sensitized Solar Cell Output Power Based on FTO Disposition Immersion Time in Papaya Leave Chlorophyll
on
Journal of Electrical, Electronics and Informatics, Vol.7 No. 1, July 2023
28
Dye Sensitized Solar Cell Output Power Based on FTO Disposition Immersion Time in Papaya Leave Chlorophyll
Poppy Diana Sari1*, I Made Anom Sutrisna Wijaya2, Ida Ayu Dwi Giriantari3, Ni Made Wartini4 and Rukmi Sari Hartati5
-
1Doctoral program of Agricultural Science. Udayana University
-
2,4Faculty of Agricultural Technology. Udayana University
-
3,5Faculty of Engineering. Udayana University
Bali -Indonesia
Abstract—Dye-Sensitized Solar Cell (DSSC) is a dye-based organic solar cell as a photon absorber developed by Prof. Gratzel (1991). Natural dyes are a solution to replace synthetic dyes which are commonly used for DSSC because they are affordable, available in large quantities, environmentally friendly and sustainable. The aims of this study was to analyze the effect of immersion time on the disposition of flourine-doped tin oxide – Titanium dioxide (FTO-TiO2) in the natural dye chlorophyll on the current, voltage and power produced. Using papaya leaves extract with a direct gap energy of 2.428 Ev, FTO-TiO2 deposition was immersed in chlorophyll with 5 levels of immersion time and 3 groupings. Current and voltage measurements were made at the output of the DSSC prototype. Using the Solar Power Generation System, the output voltage (Voc) is 341.167 mV, the current (Isc) is 64.755 μA and the output power is 1.49 x 10-5 w/cm2 at 60 hours of immersion.
Index Terms—Immersion, chlorophyll, power, DSSC
-
I. INTRODUCTION1
One alternative energy that converts solar energy into electrical energy is solar cells [1]. Solar cells are made of semiconductor materials such as silicon, titanium oxide, germanium and so on [2]. Since silicon solar cells, the first breakthrough in solar cell technology is the Dye sensitized solar cell (DSSC). According to [3], DSSC is a photoelectrochemical solar cell using an electrolyte as a charge transport medium, in contrast to conventional solar cells. DSSC is devided into several parts that are deposited between the two conductive glasses, including the elctrolyte, titanium dioxide (TiO2) nanopores, dye molecules adsorbed on the TiO2 surface and the catalysts.
According to [4], DSSC consists of a pair of glass substrates coated with Transparent Conducting Oxide (TCO) material which act as electrodes and counter electrodes that face each other and are separated by an I-/I3-
(iodide / triiodide) redox electrolyte arranged in a sandwich structure, as shown in figure 1. The TCO counter electrode is coated with a catalyst in the form of a layer of carbon or platinum to accelerate the redox reaction. Based on [5], on the electrodes a layer of porous TiO2 nanocrystals was deposited as a photoanode, and dye sensitized as photosensitizers. The electron transfer reaction is the basic working principle of DSSC which can be described through the electron transfer cycle of DSSC components, so that it can produce electrical energy [6].
At the beginning of its development, Michael Gratzel produced DSSC with an energy conversion efficiency of 7.9% using ruthenium complex synthetic dye type N3. Synthetic dyes such as ruthenium complexes are relatively expensive and the fabrication process is difficult, because of this, exploration of natural dyes is intensively carried out to find alternatives to natural chromophore compounds that have the same or even better performance than complex ruthenium dyes. Chlorophyll, carotene, xanthophyll and anthocyanins are examples of natural dyes which are the
center of study because of their ability to absorb light in the process of photosynthesis. The main property of a sensitizer is its absorption value, that is, its ability to absorb light. According to [7], some of the properties of the dye molecule as a sensitizer include panchromaticity, having a functional group that allows it to bind to the wide-gap material conduction band (TiO2), having an excitation energy level corresponding to the material's conduction band, having a redox potential energy level base and corresponding excited energy levels, has a large (positive) redox potential, has chemical and physical stability, especially stability to heat.
The advantages of using natural dyes in DSSC are the absence of side effects on health, non-toxic, and easy to obtain from nature. While the drawbacks are low pigment concentration and stability, poor color uniformity, the color spectrum is not as wide as synthetic dyes and less durable. So far, natural dyes that are often used as sensitizers are anthocyanin substances which are pigments that cause almost all red-blue colors in flowers, leaves and fruit in higher plants [8]. Natural dyes in flowers, leaves and fruit can be extracted by simple procedures, low costs, nontoxicity and complete biodegradation, natural dyes have become popular research subjects [9]. However, this does not rule out the possibility of using chlorophyll as a dye senstizer due to its abundant presence in nature.
In photosynthesis, chlorophyll plays a role in absorbing and converting light energy into chemical energy. Chlorophyll is abundant in green plants, photosynthetic bacteria and algae. The light absordbed by chlorophyll is in the form of electromagnetic radiation on the visible spectrum. Sunlight contains all the colors of the visible spectrum from red to violet, but chlorophyll is not able to absorb well all the wavelengths of its elements evenly [10]. According to [11], papaya plants have the highest total chlorophyll, chlorophyll a and chlorophyll b content compared to cassave and peanut plants. This prompted researchers to utilize papaya leaf chlorophyll as a dye sensitizer in the DSSC being studied. Chlorophyll-a and b are the strongest in absorbing light in the red section (600700 nm), while the least in green light (500-600 nm). Chlorophyll a is a complex compound between porphyrin and magnesium (Mg) which contains ring V (cyclopentanone ring). The four N atoms are linked by Mg2+ ionic bonds. Chlorophyll a has the chemical formula C55H72O5N4Mg. While chlorophyll b is bound to proteins in cells. Chlorophyll b has the chemical formula C55H70O6N4Mg.
Chlorophyll dyes have several factors that affect stability such as pH, solvent influence, light intensity, enzymes, oxidizers, and temperature used [12]. The main
chemical degradation that occurs in chlorophyll is pheophynitization, epimerization, and pyrolysis, as well as by hydroxylation, oxidation or photooxidation, if there is the influence of light [13]. The results of the research by [14,15] stated that the addition of the color stabilizer sodium bicarbonate (NaHCO3) can prevent chlorophyll degradation. NaHCO3 solution is an alkaline salt. Alkaline substances such as NaHCO3 have been used in the blanching process in green vegetables to increase the pH and maintain chlorophyll after processing [13 ; 16].
In the middle of the chlorophyll molecule there is a magnesium (Mg) atom as described by Ludin et al. [17] that in chlorophyll which has the ability to inject electrons into the semiconductor conduction band and is an atom that can bind to the surface of TiO2 thereby increasing the rate of the electron transfer reaction is Mg, so that chlorophyll is suitable when used as a dye sensitizer in DSSC. According to [1], that the immersion time of flourine-doped tin oxide – Titanium dioxide (FTO-TiO2) disposition in the dye sensitizer affects the voltage and current strength obtained. The longer the immersion time, the higher the voltage and current generated. So in this study, the researchers aimed to determine the immersion time of FTO-TiO2 disposition in papaya leaves chlorophyll which would produce a better output power.
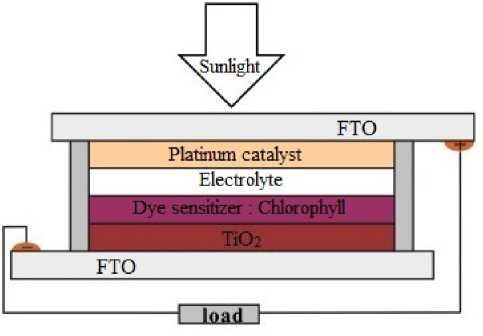
Fig. 1. Dye sensitized solar cell (DSSC) scheme
Chlorophyll extract production
In papaya leaves extract production, the Carolina papaya leaves are first processed into papaya leaves powder. The preparation of papaya leaves powder includes washing, blanching at 100 oC for 1 minute, reducing the size by 5 cm ± 0.5 cm, drying at 50 oC until the water content of the material is 8% ± 0.5%, as well as crushing and sifting to size 80 mesh (177 microns).
Chlorophyll was extracted from papaya leaves powder using maceration extraction method at room temperature. Pure analyze 96% acetone from the Merck brand was used as a solvent with a raw material ratio (papaya leaves powder): solvent 1:10 (w/v) referring to research conducted by [18]. Referring to [15], in the process of extracting chlorophyll from papaya leaves, 3% of the Merck brand pure analyze stabilizer NaHCO3 was added before extraction. Papaya leaves chlorophyll extraction was carried out for 48 hours. The extraction results were then filtered using coarse filter paper and followed by filtering using whatman filter paper size no.1 to obtain papaya leaves chlorophyll extract. The chlorophyll extract was evaporated in a rotary vacuum evaporator at a pressure of 0.18 atm and a temperature of 50 oC [15].
Electrolyte production
Compounds in the manufacture of this electrolyte solution are 3 grams of Merck pure potassium iodide (KI) analyzer and 3 ml of liquid iodine 10% solution pure analyzer Merck brand. Liquid potassium iodide and iodine are mixed using a stirrer for 30 minutes. Immersion of the FTO layer in dye
Solaronix fluorine-doped tin oxide (FTO) glass that has been coated with TiO2 is immersed in chlorophyll dye for a certain soaking time (12 hours, 24 hours, 36 hours, 48 hours and 60 hours).
Dye sensitized solar cell prototype assembly
The arrangement of the DSSC layers is in the form of glass as a substrate which has been coated with TiO2 which has been soaked in dye and is called the working electrode (anode) arranged in a sandwich with a reference electrode (cathode). The reference electrode is a fluorine-doped tin oxide (FTO) glass that has been coated with Platinum carbon paste from Solaronix. The DSSC layer that has been arranged in a sandwich manner is dripped with electrolyte solution.
The observed variables include the value of the energy gap from papaya leaf chlorophyll extract, the value of current, voltage and output power produced.
Observation of the visible light absorption spectrum of chlorophyll was carried out using a UVVis Spectrophotometer (UV-1800 Shimadzu). The chlorophyll gap energy calculated using the Tauc Plot equation, namely:
(α∕ιυ)n = A{hυ — Eg) (1)
According to Suprasetyo and Setiarso [19], respectively α, h, Ev and A are the absorption coefficient, planck's constant, energy gap and constant, while the exponent n depends on the nature of the transition. The amount of energy required for an electron to get excited from the
HOMO state to the LUMO state is indicated by the energy gap.
The current and voltage characterization of the DSSC layer was carried out using a voltmeter (V) and ammeter (A). The light is directed perpendicular to the surface of the cell. The test was carried out using halogen light. The correct setting for measuring I-V on a solar cell is based on a 4-wire junction, known as the Kelvin configuration [20]. Testing the current and voltage using the Woosun brand Solar Power Generation System type WS-SCO3P.
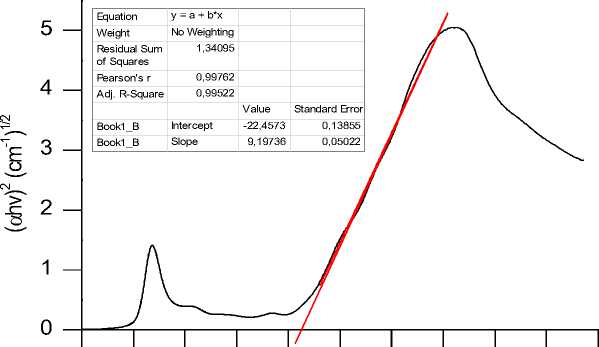
The results of the indirect chlorophyll energy gap analysis using the Tauc plot analysis are shown in figure 1 and an energy gap of 2.442 eV is obtained, while the direct chlorophyll energy gap is 2.428 Ev as shown in Fig. 2. Based on [15] and [21] research, the energy gap of the anatase phase TiO2 semiconductor is 3.2 eV, because the chlorophylls energy gap in this study is smaller than the energy gap of the anatase phase TiO2 semiconductor, it means that chlorophyll can be used as a dye sensitizer.
This shows that papaya leaves chlorophyll can be used as a DSSC dye sensitizer. Electrons in chlorophyll will be excited from the HOMO state to the LUMO state when absorbing photon energy from visible light, electrons then move from the TiO2 semiconductor to the conduction band and diffuse to the ITO (Indium Tin Oxide) substrate [22].
From the research results, the current and voltage are measured using the Solar Power Generation System, the results are obtained in accordance with Table 1.
Based on the data presented in table 1, it shows that the longer the process of immersing the FTO disposition in chlorophyll dye, the higher the output power produced from the DSSC prototype. In this study, the best results were obtained with an output power of 4.15 x 10-5 w/cm2 in the 60 hour treatment of FTO disposition immersion in papaya leaves chlorophyll. In line with [1], that immersion time affects the voltage and current strength obtained by DSSC. The longer the immersion, the higher the voltage and current generated. With a maximum voltage value (Vmpp) of 0.3402 V and a fill factor of 0.6761, this study produced better results than the research [23], which used dragon fruit peel anthocyanin as a dye sensitizer to produce Vmpp of 0.31 V and the highest fill factor of 0.480. This research also produced an effective output voltage of 0.1736 V. The longer the immersion of the FTO-TiO2 disposition in
chlorophyll, the higher the current and voltage generated. This is due to the longer immersion time causing the greater number of chlorophyll molecules to be adsorbed on the surface of the TiO2 thin film so that the absorption of
photons is also greater. The more photons absorbed, the greater the number of excited dye molecules and the greater the number of electrons injected into the TiO2 thin layer.
1,6 1,8 2,0 2,2 2,4 2,6 2,8 3,0 3,2 3,4 3,6 Energi (eV)
Fig. 2. Indirect energy gap of papaya leaves chlorophyll
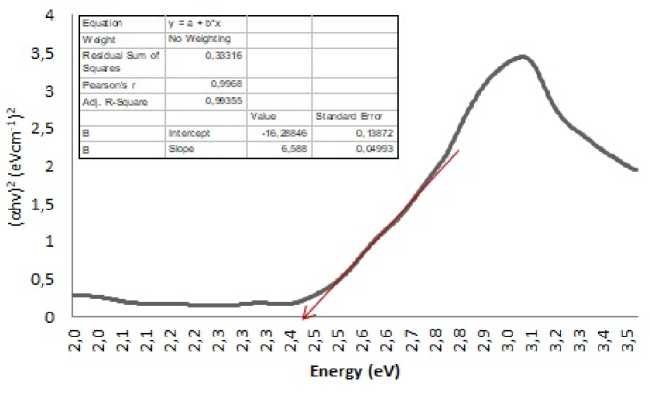
Fig. 3. Direct Energy gap of papaya leaves chlorophyll
Table 1
The output power of the DSSC prototype
|
Immersion time Hours |
Isc A |
Impp A |
Voc V |
Vmpp V |
Jsc A/cm2 |
FF |
Pmax W/cm2 |
|
12 jam |
3.245 x 10-5 |
2.2 x 10-5 |
0.172 |
0.171 |
9.01 x 10-5 |
0.671 |
1.04 x 10-5 |
|
24 jam |
3.303 x 10-5 |
2.24 x 10-5 |
0.175 |
0.174 |
9.17 x 10-5 |
0.672 |
1.08 x 10-5 |
|
36 jam |
5.731 x 10-5 |
3.89 x 10-5 |
0.303 |
0.301 |
1.59 x 10-4 |
0.675 |
3.25 x 10-5 |
|
48 jam |
6.092 x 10-5 |
4.13 x 10-5 |
0.321 |
0.32 |
1.69 x 10-4 |
0.676 |
3.67 x 10-5 |
|
60 jam |
6.475 x 10-5 |
4.39 x 10-5 |
0.341 |
0.34 |
1.80 x 10-4 |
0.676 |
4.15 x 10-5 |
The output power of the DSSC prototype by utilizing papaya leaves chlorophyll as a dye sensitizer was tested by immersion time of FTO disposition in chlorophyll dye. The highest output power was obtained at 4.15 x 10-5 w/cm2 in chlorophyll dye with a direct gap energy of 2.428 Ev for 60 hours of immersion.
References
-
[1] Susmiyanto, D., Wibowo, N.A. dan Sutresno, A. Fabrikasi Sel Surya Pewarna Tersensitisasi (SPPT) dengan Memanfaatkan Ekstrak Antosianin Ubi Jalar Ungu (Ipomoea batatas L). (Fabrication of Dye Sensitized Solar Cells (SPPT) Utilizing Purple Sweet Potato Anthocyanin Extract (Ipomoea batatas L)). Prosiding Seminar Nasional Sains dan Pendidikan Sains VIII UKSW. Vol. 4 (1) : 104-105. 2013.
-
[2] McDonald, S.A., Konstantatos, G., Zhang, S., Cyr, P.W., Klem, E.J., Levina, L., dan Sargent, E.H. Solution-processed PbS quantum dot infrared photodetectors and photovoltaics. Nature Materials Vol. 4 (2) : 138-142. 2005.
-
[3] Smestad, GP, Gratzel, M. Demonstrating electron transfer and nanotechnology: Aa natural dye-sensitized nanochrystalline energy converter. Journal of Chemical Education, 75 (6), 752-756. 1998.
-
[4] Giusti, M. M. and R. E. Wrolstad. Characterization and measurement of anthocyanin by UV-visible
spectroscopy. In R.E. Wrostald TE, Acree EA, Dekker MH. Penner DS, Reid SJ, Schwarrtz CF, Shoemaker D, Smith PS (eds). Handbook of Food Analytical Chemistry: Pigmens, Colorants, Flavors, Texture, and Bioactive Food Components, Hoboken. New Jersey. John Wiley Sons. pp 624. 2001.
-
[5] Maddu A, Zuhri M dan Irmansyah. Penggunaan ekstrak antosianin kol merah sebagai fotosensitizer pada Sel Surya TiO2 nanokristal tersensitisasi dye. (Use of red cabbage anthocyanin extract as a photosensitizer in dye-sensitized TiO2 nanocrystalline solar cells). Makara Teknologi Vol. 11, No. 2. 2007.
-
[6] Kumara, M.S.W. and Gontjang, P. Studi Awal Fabrikasi Dye Sensitized Solar Cell (DSSC) dengan Menggunakan Ekstraksi Daun Bayam (Amaranthus hybridus l) sebagai Dye Sensitizer dengan Variasi Jarak Sumber Cahaya pada DSSC. (Preliminary Study of Fabrication of Dye Sensitized Solar Cell (DSSC) Using Spinach Leaves Extract (Amaranthus hybridus l) as Dye Sensitizer with Variation of Light Source Distance
on DSSC). Surabaya: Institut Teknologi Sepuluh November. hal: 111. 2012.
-
[7] Halme, J. Dye-sensitized nanostructured and organic photovoltaic cells: technical review and preliminary tests. Master’s theses. Department of Engineering Physics and Mathematics, Helsinki University of Technology, Espoo. 2002.
-
[8] Harborne, J.B. Metode fitokimia: Penuntun cara modern menganalisis tumbuhan. (Phytochemical methods: A guide to the modern way of analyzing plants). Institut Teknologi Bandung, Bandung. 1996. http://repository.ipb.ac.id/. Diakses pada Tanggal 12 April 2020.
-
[9] Latif,A., Monzir,S., Mahmoud, B. Dye-sensitized solar cells using dyes extracted from flowers, leaves, parks and roots of three trees. International Journal of Renewable Energy Research. Vol.5, No.1. 2015.
-
[10] Gobel, R.B., E. Johannes dan A.I. Latunra. Biologi Dasar. (Basic biology). Program TPB-UNHAS. Makassar. 2006.
-
[11] Abdilah, F., Indah, R., Ahyar, A. Pengujian Daya Antioksidan dan Sifat Toksisitas Ekstrak Co(II) Turunan Klorofil. (Antioxidant Analyses and Toxicity Properties of Chlorophyll Derivative Co(II) Extract). Jurnal MIPA. 2014.
-
[12] Vila, Marta M.D.C., Marco V. Chaud, and Victor M. Balcão. Microencapsulation of natural antioxidant pigments in microencapsulation and microspheres for food applications. Editor L. M. C. Sagis, Academic Press, London, 369-390. 2015.
-
[13] Koca, Nuray, Feryal Karadeniz, and Hande Selen Burdurlu. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chemistry. United Kingdom. Volume 100, 609–615. 2007. https://doi.org/10.1016/j.foodchem.2005.09.079.
-
[14] Markus P. K., Widodo F. M. dan Tri W. W. Pengaruh Penambahan MgCO3 dan NaHCO3 dengan Pernbedaan Pencahayaan Terhadap Stabilitas Pigmen Klorofil a Mikroalga. (The Effect of Addition of MgCO3 and NaHCO3 with Differences in Lighting on the Stability of Microalgae Chlorophyll a Pigment). Universitas Diponegoro. Semarang. 2013.
-
[15] Aryanti, N., A. Nafiunisa, F. M. Willis. Ekstraksi dan karakterisasi klorofil dari daun suji (Pleomele angustifolia) sebagai pewarna pangan alami. (Extraction and characterization of chlorophyll from suji leaves (Pleomele angustifolia) as a natural food coloring). Jurnal Aplikasi Teknologi Pangan 5 (4). Indonesian Food Technologists. 2016. https://doi.org/10.17728/jatp.183.
-
[16] Srilakhsmi, B. Food Science, 3rd ed., New Age International, New Delhi, pp.171-211. 2003.
-
[17] Ludin, N.A., Al-Alwani Mahmoud, A.M., Bakar Mohamad, A., Kadhum, A.A.H., Sopian, K., Abdul Karim, N.S. Review on the development of natural dye photosensitizer for dye-sensitized solar cells.
Renewable and Sustainable Energy Reviews. Volume 31, page 386–396. 2014.
https://doi.org/10.1016/j.rser.2013.12.001.
-
[18] Setiari, Nintya and Nurchayati, Yulita. Eksplorasi kandungan klorofil pada beberapa sayuran hijau sebagai alternatif bahan dasar food supplement. (Exploration of chlorophyll content in some green vegetables as an alternative food supplement base material). Bioma Journal. Indonesia. Volume 11, no.1. pp. 6-10. 2009. https://doi.org/10.14710/bioma.11.1.6-10.
-
[19] Suprasetyo, A., P Setiarso. Pembuatan elektroda pasta karbon termodifikasi zeolit untuk analisis fenol secara cyclic stripping voltammetry. (Preparation of zeolite modified carbon paste electrodes for phenol analysis by cyclic stripping voltammetry). UNESA Journal of Chemistry. Vol. 5, no.3. Page 86-93. 2016.
-
[20] Behrang H. Hamadani and Brian Dougherty. Solar Cell Characterization. in M.P. Paranthaman et al. (ed.), Semiconductor Materials for Solar Photovoltaic Cells, Springer Series in Materials Science 218. 2016. DOI 10.1007/978-3-319- 20331-7_8.
-
[21] Ganta D, Jara J, Villanueva R. Dye-sensitized solar cells using aloe vera and cladode of cactus extracts as natural sensitizers. Chemical Physics Letter Journal. Netherlands. Volume 679: page 97–101. 2017. https://doi.org/10.1016/j.cplett.2017.04.094.
-
[22] Hikmah, Irmayatul dan Gontjang Prajitno. Pengaruh Penggunaan Gel-Electrolyte pada Prototipe Dye Sensitized Solar Cell (DSSC) berbasis TiO2 Nanopartikel dengan Ekstrak Murbei (Morus) sebagai Dye Sensitizer pada Substrat Kaca ITO. (Effect of Using Gel-Electrolyte on Dye Sensitized Solar Cell (DSSC) Prototypes based on TiO2 Nanoparticles with Mulberry Extract (Morus) as Dye Sensitizer on ITO Glass Substrates). Science and Art Journal. Institute Teknologi Sepuluh November. Surabaya – Indonesia. Volume 4, No 1. 2015. https://doi/
10.12962/j23373520.v4i1.8655.
-
[23] Setiawan, I.N. Konversi sinar matahari menjadi energi listrik oleh dye sensitized solar cell (DSSC) menggunakan pewarna alami yang diekstrak dari komponen bioaktif limbah buah. (Conversion of sunlight into electrical energy by dye sensitized solar cells (DSSC) using natural dyes extracted from the
bioactive components of fruit waste). Disertasi Fakultas Teknik – Universitas Udayana. Bali. 2020.
-
[24] Fitriya, H., Handayani, R. D., Lesmono, A. D. Pengaruh lama perendaman TiO2 dalam dye sensitizer ekstrak daun tembakau (Nicotiana tabacum L) terhadap efisiensi dye sensitizer solar cell (DSSC). (Effect of TiO2 immersion time in tobacco leaf extract dye sensitizer (Nicotiana tabacum L) on dye sensitizer solar cell (DSSC) efficiency). Jurnal Pembelajaran Fisika, Vol. 5 No. 4, hal 343 – 350. 2017.
Discussion and feedback