TEMPERATURE OPTIMIZATION AGAINST P-METHOXYCINAMIC ACID SYNTHESIS THROUGH ULTRASONIC WAVE-ASSISTED KNOEVENAGEL CONDENSATION
on
JURNAL KIMIA (JOURNAL OF CHEMISTRY) 16 (1), JANUARI 2022
p-ISSN 1907-9850
DOI: https://doi.org/10.24843/JCHEM.2022.v16.i01.p13
e-ISSN 2599-2740
TEMPERATURE OPTIMIZATION AGAINST P-METHOXYCINAMIC ACID SYNTHESIS THROUGH ULTRASONIC WAVE-ASSISTED KNOEVENAGEL CONDENSATION
E. Indriyanti*, M. Suryaning P, Y. Purwaningsih dan F. X. Sulistiyanto
Program Studi Farmasi, Sekolah Tinggi Ilmu Farmasi Yayasam Pharmasi, Semarang, Jawa Tengah, Indonesia
*Email: erwinindriyanti05@gmail.com
ABSTRACT
Cinnamic acid derivative compounds are found in almost all plants but the quantity is very small that it cannot only rely on the results collected from the extraction method or the isolation of plant parts alone. Increasing amount of production of cinnamic acid derivatives can be done by chemical synthesis. One of the cinnamic acid derivatives that can be synthesized is p-methoxycinamic acid. It is a derivative of cinnamic acid that is substituted by a methoxy group at para position. The synthesis of this cinnamic derivative was obtained through the knoevenagel condensation reaction with the sonochemical method by reacting 6.61 mmol anisaldehyde, 16.8 mmol malonic acid, and 1.12 mmol β-alanine dissolved in 37.1 mmol pyridine in an Erlenmeyer flask, then sonicated for 60 minutes at temperatures (400C, 500C, 600C). The synthesized compound was tested organoleptically and its melting point was measured. The result structure was elucidated using FTIR and GC-MS. The synthesized compound in the form of shiny white fine crystals had a distinctive odor and an optimum temperature of 600C and produces % yield of 92.71%. The results of the structural elucidation test of the synthesized compound using FTIR-ATR showed the presence of an OH carboxylate group, C=O carboxylate, C=C, an aromatic group, C=C conjugated aromatic group, and aromatic substitution in the para position. Testing by GC-MS found that the compound has a purity of 100% with a retention time of 11.71 minutes, with a base peak of 178 m/z with a relative abundance of 100%.
Keywords: elucidation, knoevenagel, p-methoxycinamic acid, synthesis, sonochemical.
ABSTRAK
Senyawa-senyawa turunan asam sinamat terdapat hampir di semua tanaman namun kuantitasnya sangat kecil sehingga tidak bisa hanya mengandalkan hasil-hasil yang dikumpulkan dari metode ekstraksi ataupun isolasi bagian tanaman saja. Peningkatan jumlah produksi dari senyawa-senyawa turunan asam sinamat dapat dilakukan dengan sintesis kimia. Salah satu turunan asam sinamat yang dapat disintesis adalah asam p-metoksisinamat. Senyawa asam p-metoksisinamat merupakan senyawa turunan dari asam sinamat yang tersubstitusi gugus metoksi pada posisi para. Sintesis turunan sinamat ini didapatkan melalui reaksi kondensasi knoevenagel dengan metode sonokimia dengan mereaksikan 6,61 mmol anisaldehid, asam malonat 16,8 mmol, dan β-alanine 1,12 mmol dilarutkan dalam piridin 37,1 mmol dalam labu Erlenmeyer, kemudian disonikasi selama 60 menit pada suhu (400C, 500C, 600C). Senyawa hasil sintesis diuji organoleptis dan diukur titik leburnya. Struktur hasil dielusidasi menggunakan FT-IR dan GC-MS. Senyawa hasil sintesis berupa Kristal halus berwarna putih mengkilap memiliki bau khas dan suhu optimum 60oC dan menghasilkan % yield sebesar 92,71%. Hasil Uji elusidasi struktur menggunakan FTIR-ATR dari senyawa hasil sintesis menunjukkan adanya gugus OH karboksilat, C=O karboksilat, C=C, gugus aromatik, C=C terkonjugasi gugus aromatik, dan substitusi aromatik di posisi para. Pengujian dengan GC-MS didapatkan senyawa memiliki kemurnian 100% dengan waktu retensi 11,71 menit, dengan base peak 178 m/z dengan kelimpahan relatif 100%.
Kata kunci: asam p-metoksisinamat, elusidasi, knoevenagel, sintesis, sonokimia.
INTRODUCTION
Cinnamic acid is an intermediate with significant potential for the synthesis of several important industrial chemicals (Pawar, Wagh and Lali, 2016). Cinnamic acid and its derivatives play a vital role in the synthesis of
other important compounds and as a precursor for the synthesis of commercial cinnamic esters which are important in the perfume, cosmetic and pharmaceutical industries (Indriyanti and Prahasiwi, 2020). Cinnamic acid and its derivatives are known to have many biological activities, are used as active ingredients in
sunscreen and bleach preparations and are used in the synthesis of macromolecules as a very important building block for various classes of polymers, have interesting properties, especially high photoreactivity due to their presence in the main chain. or side, from the sinamoyl group (Chiriac, Tanasa and Onciu, 2005a), (Guzman, 2014) (Ekowati et al., 2019).
Cinnamic acid derivative compounds are found in almost all plants but the quantity is so small that it cannot only rely on the results collected from the extraction method or the isolation of plant parts alone. Increasing the amount of production of cinnamic acid derivatives can be done by chemical synthesis (Kadidae et al., 2020). One of the cinnamic acid derivatives that can be synthesized is p-methoxycinamic acid. P-methoxycinamic acid is a derivative of cinnamic acid which is substituted by a methoxy group in the para position (Sharma, 2011). P-methoxycinamic acid is known to be a compound that functions as an analgesic and anti-inflammatory,(Pawar, Wagh and Lali, 2016) antioxidant, antidiabetic and hepatoprotective, neuroprotective and chemopreventive activity (Lipase et al., 2020).
The synthesis of cinnamic derivatives can be carried out in several ways, namely the perkin, knoevenagel, wittig and reformatsky
reactions(Ekowati et al., 2010), but in this study the knoevenagel condensation reaction was selected because it fulfills the requirements used in the synthesis and can provide large yields. The synthesis of cinnamic derivates via knoevenagel condensation has wide applications in cosmetics, fragrances and pharmaceuticals (Pawar, Wagh and Lali, 2016) Several cinnamic acid ester derivatives have been shown to be antitumor, anti-inflammatory and sunscreen. Conventionally, cinnamic acid derivatives are synthesized with a combination of aromatic aldehydes and active methylene compounds with organic / inorganic bases. The condensation of aldehydes and malonic acid in several ester derivatives has been widely applied in the pharmaceutical field (Ghomi and Akbarzadeh, 2018)
Knoevenagel condensation is a nucleophilic addition reaction, between an aldehyde and a compound that has hydrogen against two activating groups (eg C = O), using ammonia or amine as a catalyst. Some of the factors that influence the reaction are the substituent attached to the aldehyde group, the availability of the active methylene group and the catalyst used. The Knoevenagel reaction is primarily used for aromatic aldehydes.
PhCHO
CH2(CO2H)2 -B!B⅛
PhCH—CH(CO2H)2
[ PhCH=C(CO2H)2 I
OH
-CO2 ----- PhCH=CHCO2H
Figure 1. Knoevenagel's Condensation Reaction
Synthesis of p-methoxycinamic acid has been using reflux methods. Synthesis by reflux method requires high temperatures and a long time. Synthesis by reflux method has been carried out in the synthesis of 4-methoxycinamic acid at a temperature of 120˚C. The synthesis of cinnamic acid by Chiriac, Tanasa and Nechifor (2009) requires a temperature of 1800C-1900C for 9-12 hours,(Chiriac, Tanasa and Onciu, 2005b) takes 9 hours. The length of time and the high temperature are the shortcomings of conventional methods, so there needs to be an alternative method in the synthesis of cinnamic acid and its derivatives that can overcome the shortcomings of conventional methods, namely the sonochemical method.
The use of ultrasonic waves in organic synthesis has become an option in recent years. The sonochemical effect is cavity (Cavity). Ultrasonic waves occur at a frequency of 20 KHz to 100 MHz. Ultrasonic waves are known to accelerate various types of organic reactions and are believed to be an important technique in organic synthesis (Li et al., 2001) Sonochemistry offers short reaction times and high yields in the synthesis of organic compounds (Babu, Devi and Dubey, 2013), it is environmentally friendly because it minimizes waste and minimizes energy use (Patel et al., 2014).
The starting materials used in the synthesis of p-methoxycinamic acid this time are p-anisaldehyde, malonic acid, β-alanine
with pyridine catalyst. The choice of ingredients in the synthesis of p-methoxycinamic acid was based on research conducted by Verley-Dobner with a slight modification (Hoai et al., 2018).
Based on this description, this research was conducted to obtain p-methoxycinamic acid through knoevenagel condensation with the help of ultrasonic waves and determine the optimal temperature in the synthesis.
MATERIALS AND METHODS
Materials
The materials used in this study were p-anisaldehyde (pa, Sigma-Aldrich), malonic acid (pa, Merck), β-alanine (pa, Merck), pyridine (pa, Merck), NaHCO3 (pa, Merck), aquadest , hydrochloric acid (pa, Merck), diethyl ether (pa, Merck), ethanol (pa).
The equipment used in this study were analytical scales, measuring cups, beaker glass, erlenmeyer, spatula, stirring rod, dropper pipette, watch glass, buchner funnel, vacuum pump, basin, statif, clamps, aluminum foil, Whattman filter paper, separating funnel. , pH indicator paper, melting point test kit, sonicator (BRANSON 1510 ultrasonic cleaning bath, 45 kHz), oven, FT-IR (Agilent Technologies Cary 630 FTIR), GC-MS (Shimadzu QP 2010) in the UII campus laboratory.
Methodes
p-Methoxycinamic Acid Synthesis
The APMS synthesis uses a procedure based on Verley-Dobner [16] with minor modifications. A total of 6.61 mmol of anisaldehyde, 16.8 mmol of malonic acid, and 1.12 mmol of β-alanine were dissolved in 37.1 mmol pyridine in an Erlenmeyer flask. The solution is covered with aluminum foil, then sonicated for 60 minutes at temperatures (400C, 500C, 600C). After that it is cooled to room temperature in an ice bath, then as much as 8 mL of HCl is added slowly until a white precipitate is formed. APMS solids are filtered with a vacuum pump and rinsed with cold distilled water. The solid obtained was recrystallized using a mixture of distilled water : ethanol (3: 1). The crystals formed are then dried in an oven to dry. After drying, the
crystals were weighed and the yield was calculated, then the synthesized compounds were tested for their melting point, solubility, FTIR-ATR and GC-MS spectrophotometers.
Data Analysis
Analysis of p-methoxycinamic acid was carried out using FTIR ATR and GS-MS, while the measurement of the percentage yield of p-methoxycinamic acid was carried out using the following formula :
. , , synthesis yield
% yield =
theoretical weight
x 100%
FTIR-ATR analysis is used to determine the structural formulas of compounds, while GC-MS analysis is used to identify compounds as determinants of molecular weight and determination of molecular formulas, and determine the level of purity of the resulting compounds.
RESULTS AND DISCUSSION
The synthesis of p-methoxycinamic acid in this study was carried out through knoevenagel condensation between anisaldehyde, malonic acid and β-alanine using a pyridine catalyst (Hoai et al., 2018) through ultrasonic wave-assisted sonochemical method (Ghomi and Akbarzadeh, 2018) The use of knoevenagel condensation refers to Verley-Doebner with slight modifications. Knoevenagel condensation using malonic esters, malonic acid or acetic acid in place of malonic anhydride in order to produce good yields of cinnamic acid derivatives with electron donor substituents at the para position in the aromatic ring (Hoai et al., 2018). The use of malonic acid and β-alanine and the presence of pyridine as a catalyst shows great potential in the preparation of cinnamic acid derivatives (Hoai et al., 2018). The use of pyridine as an organic base catalyst is a tertiary aromatic amine with sp2 hybridization, the lone pair of nitrogen pyridine is more attracted to the aromatic ring so that its basicity and nucleophyllan properties are rather low (Ekowati, et. al, 2010).
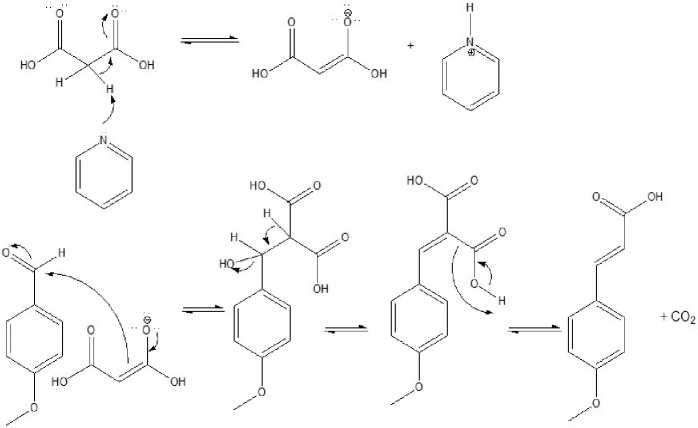
Figure 2. The condensation mechanism of Knoevenagel malonic acid and anisaldehyde
The mixture of anisaldehyde, malonic acid and β-alanine using a pyridine catalyst was subjected to the sonication process for 60 minutes with 3 different temperature variations, namely 400C; 500C; 600C. The choice of sonication timing was based on research conducted by Indriyanti and Prahasiwi (2020). Temperature variations were carried out to determine the optimal temperature for knoevenagel condensation with ultrasonic waves which produced the largest % yield. This was done because temperature can affect the likelihood of collisions between molecules, where the higher the temperature in the reaction the higher the kinetic energy produced by compound molecules which will result in more collisions between molecules. This phenomenon will increase the reaction rate and affect the reaction result (Ekowati et al., 2019). The optimum temperature was reached at 600C with a yield of 92.71%. The increase in sonication temperature results in an increase in% yield, this is because the higher the sonication temperature will result in greater kinetic energy received by molecules and collisions between molecules to start chemical reactions (Ekowati et al., 2019).
The use of ultrasonic waves in organic synthesis has become an option in recent years. The sonochemical effect is cavity (Cavity). Ultrasonic waves occur at a frequency of 20 kHz to 100 MHz. Ultrasonic waves are known to accelerate various types of organic reactions and are believed to be an important
technique in organic synthesis. Sonochemistry offers short reaction times and high yields in the synthesis of organic compounds (Babu, Devi and Dubey, 2013), it is environmentally friendly because it minimizes waste and minimizes energy use (Patel et al., 2014)
The addition of concentrated HCl in the reaction caused the formation of a white precipitate as the beginning of the formation of crystals. This process went through the salting out mechanism which decreased the solubility of the reaction compound in the solvent due to the formation of the amine salt-HCl (pyridine-HCl) that dissolved in water. This amine base worked as a solvent as well as a catalyst (Juni et. al, 2010; Hoai et al., 2018). The resultant precipitate is filtered, while saturating it with HCl. This saturation is done to form the HCl salt from the catalyst, so that the crystals are free from residual (Juni et. al, 2010). Furthermore, washing with cold aquadest was carried out with the aim of removing the remaining pyridine-HCl and malonic acid. Then recrystallization was carried out with ethanol solvent.
The results of the organoleptic test showed that the resulting synthesized compound was in the form of shiny white fine crystals with a distinctive odor and when compared to the standard p-methoxycinamic acid, the synthesized compound had the same melting point of 1690C-1720C (Mufidah, 2014) can be seen in Figure 3.

Figure 3. p-methoxycinamic acid crystals
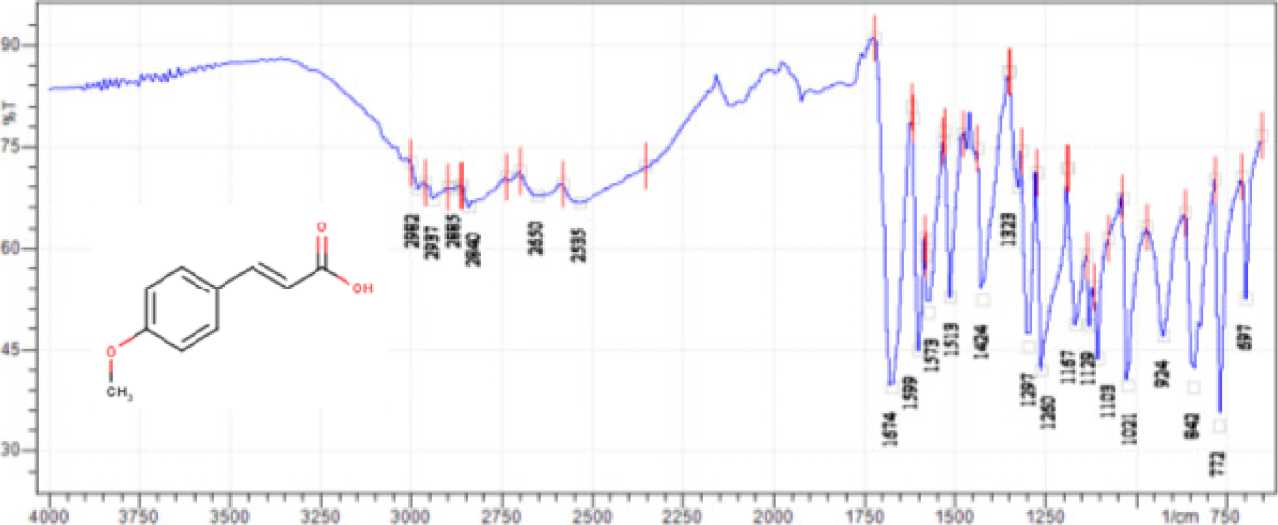
The structure elucidation test using FTIR spectrophotometry can be seen in Figure 4. The FTIR spectrum of the synthesized compound showed that the OH group uptake was at 2822 cm-1, which, when compared to the research of (Mumpuni, L and Nurhidayati, 2010), was in the range of 3006.82-2516.93cm-1 which is characterized by a wide absorption area. The C= O caroxylate group of the synthesized compound is found in the wave number 1677cm-1, which occurs at the wave number 1680cm-1. The absorption for the C=C group occurs at the wave number 1599cm-1 which is similar to the absorption for the C=C group in the study of (Pratiwi et al., 2018)
which occurred at 1590 cm-1. The C=C group conjugated with an aromatic group occurred at wave number 1573cm-1, where in the study of (Mumpuni, L and Nurhidayati, 2010) the C=C absorption conjugated with an aromatic group occurred at 1625.88cm-1.The aromatic group absorption is shown at the wave number 1513cm-1, this absorption has similarities to the aromatic group absorption in the study of which was found (Mumpuni, L and Nurhidayati, 2010) at 1514 cm-1. The substitution of the aromatic group at the para position is indicated by the appearance of a peak at the wave number 842 cm-1, this absorption is similar to the research of (Mumpuni, L and Nurhidayati, 2010) which is at 827 cm-1. The absorption range for substitution of aromatic groups at the para position is 800-860 cm-1 (Fessenden and Fessenden, 1986). The results of the elucidation using the FTIR-ATR instrument showed that the synthesized compound had a similar functional group structure to p-methoxycinamic acid. The characterization using GC-MS was carried out with the aim of ensuring the presence of target compounds in the synthesized product. The chromatogram of the synthesized compound can be seen in Figure 5.
TITLE: APMS 60 60.4/17/20201 56 PM
Figure 4. FTIR-ATR Spectra of Synthesized Compounds
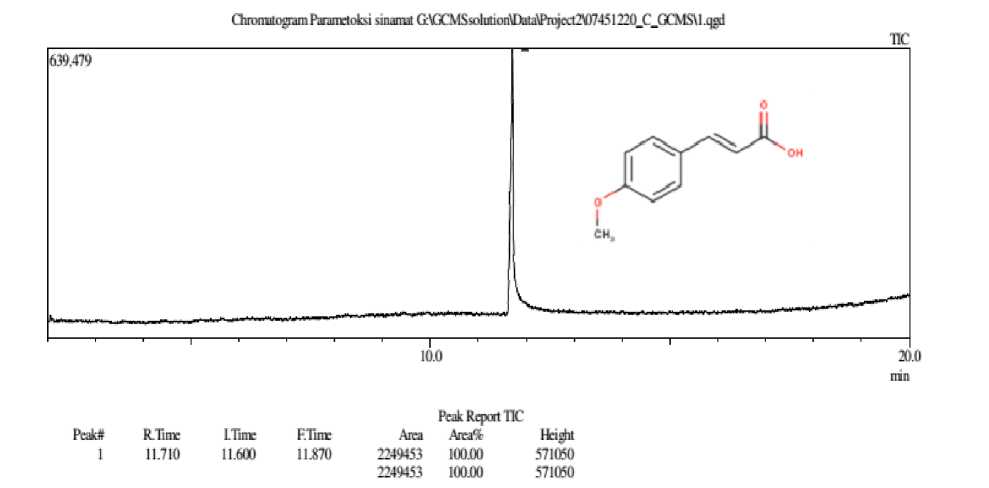
Figure 5. Chromatogram of P-methoxycinamic Acid Compound
The chromatogram pattern above showes that the synthesized compound contains one compound which is indicated by the presence of one peak. The spectral pattern formed has a retention time of 11,710 minutes, the abundance of the compound is 100%. The gas chromatography analysis showes that the pure synthesized compound is marked by the presence or appearance of one peak which indicates the absence of impurities.
The mass spectral analysis shows that the compound is p-methoxycinamic acid which has a molecular weight of 178 g / mol. The peak with m/z value of 178 is the base peak which has a relative abundance of 100%.
The fragmentation pattern of p-methoxycinamic acid can be seen in Figure 6. In the first fragmentation pattern, molecular ions release OH radicals to produce a peak with an value of m/z 161. In the second fragmentation pattern, molecular ions release COOH radicals to produce a peak with a value
of m/z 133. The third fragmentation pattern, molecular ions release CH=CH-COOH radicals to produce peak with a value of m/z 107. The fourth fragmentation pattern, the third fragmentation product releases CH3O radical ions to produce a peak with an value of m/z 77. The fifth fragmentation pattern, the fourth fragmentary compound releases CH2 radical ions to produce a peak with a value of m/z 63. The sixth fragmentation pattern, the fifth fragment released the CH=CH radical ion to produce a peak with a value of m/z 41.
The results of the analysis that have been carried out show conformity with the literature. This study succeeded in synthesizing p-methoxycinamic acid from anisaldehyde, malonic acid, β-alanine and pyridine catalyst through knoevenagel condensation with the help of ultrasonic waves with an optimum temperature of 60 °C and resulted % yield of 92.71%.
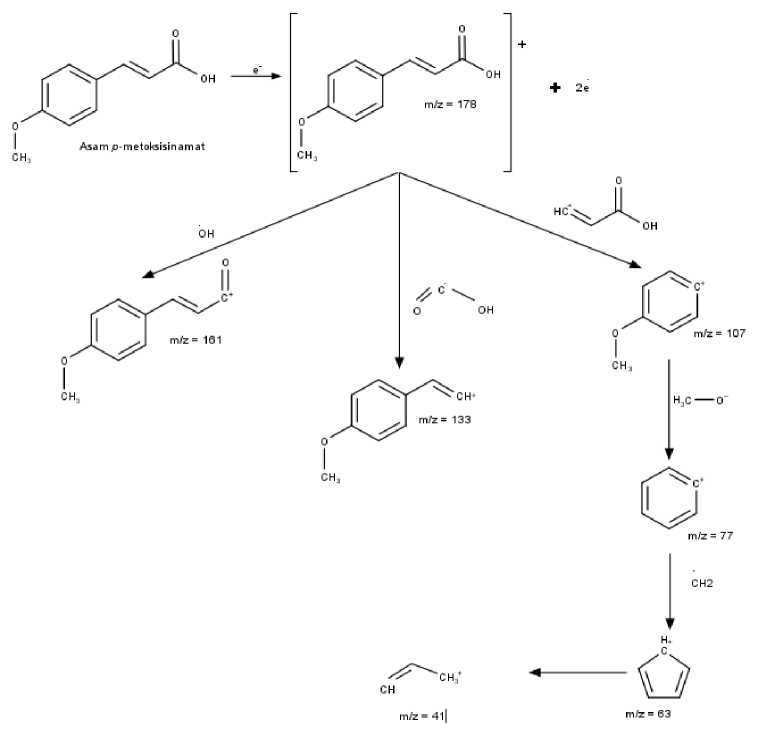
Figure 6. Fragmentation of p-methoxycinamic Acid Compounds
CONCLUSION
REFERENCES
In this research, p-methoxycinamic acid can be synthesized through knoevenagel condensation assisted by ultrasonic waves with an optimum temperature of 600C and % yield of 92.71%. The results of the FT-IR and GC-MS tests also showed that the synthesized compounds had a similar functional group structure with p-Methoxycinamic Acid
ACKNOWLEDGEMENT
Thank you to the Pharmasi Foundation and LPPM STIFAR "Semarang Pharmasi Foundation" who have provided funding for this research, along with the parties involved and who have shaped this research
Babu, P. N. K., Devi, B. R. and Dubey, P. K. 2013. Ultrasound assisted convenient, rapid and environmentally benign synthesis of N-alkylbenzimidazoles. Der Chemica Sinica. 4(1): 105–110.
Chiriac, C. I., Tanasa, F. and Nechifor, M. 2009. .A novel direct boron-mediated synthesis of cinnamic acids from aromatic aldehydes and aliphatic
carboxylic acids. Revue Roumaine de Chimie. 54(11–12): 987–991.
Chiriac, C. I., Tanasa, F. and Onciu, M. 2005a. ‘A novel approach in cinnamic acid synthesis: Direct synthesis of cinnamic acids from aromatic aldehydes and aliphatic carboxylic acids in the
presence of boron tribromide. Molecules. 10(2): 481–487. doi:
10.3390/10020481.
Chiriac, C. I., Tanasa, F. and Onciu, M. 2005b. A novel approach in cinnamic acid synthesis: Direct synthesis of cinnamic acids from aromatic aldehydes and aliphatic carboxylic acids in the presence of boron tribromide. Molecules. Molecular Diversity Preservation International, 10(2): 481– 487. doi: 10.3390/10020481.
Ekowati, J., Nuzul W. D., Astika, G. N., Budiarti, T. 2010. Pengaruh Katalis pada Sintesis Asam O-Metoksisinamat dengan Material Awal O-Metoksi Bezaldehide dan Uji Aktivitas
Analgesiknya. Majalah Farmasi
Irlangga. 8(2).
Ekowati, J., Pratama, R. P., Novianti, K. A., Diyah, N. W. 2019. The Temperature Effect on Ultrasonic-assisted of Synthesis Methyl Ferulate and Its Antiplatelet Assay. ALCHEMY Jurnal Penelitian Kimia. 15(2):. 272-286.
Fessenden, R. . and Fessenden, J. S. 1986 Kimia Organik Dasar. Ed. ke-3.. Edited by A. H. Pudjaatmaka. Jakarta: Erlangga.
Ghomi, J. S. and Akbarzadeh, Z. 2018. Ultrasonic accelerated Knoevenagel condensation by magnetically recoverable MgFe2O4 nanocatalyst: A rapid and green synthesis of coumarins under solvent-free conditions. Ultrasonics Sonochemistry. 40: 78–83. doi: 10.1016/j.ultsonch.2017.06.022.
Guzman, J. D. 2014. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules. MDPI AG. 19292–19349. doi: 10.3390/molecules191219292.
Hoai, N. T. et al. 2018. Effect of β-alanine on the preparation of 4-ethoxy-cinnamic acid. Open Materials Science Journal. 12(1): 58–67. doi:
10.2174/1874088X01812010058.
Indriyanti, E. and Prahasiwi, M. S. 2020. Synthesis of Cinnamic Acid Based on Perkin Reaction using Sonochemical Method And Its Potential as
Photoprotective Agent. 5(1): 54–61.
Kadidae, L. O., Ruslin, R., Nurliana, L., Kadir, L.A. 2020. Sintesis Ester Sinamat Menggunakan Variasi Katalis Asam. J. Pijar MIPA. 15(3): 240–246. doi: 10.29303/jpm.v15i3.1904.
Li, J. T. et al. 2001. Synthesis of cinnamic acids catalyzed by expansive graphite under ultrasound. Synthetic Communications. 31(5): 653–656. doi: 10.1081/SCC-100103251.
Mufidah, S. 2014. Modifikasi Struktur Senyawa Etil p-metoksisinamat yang Diisolasi dari Kencur (Kaempferia galanga Linn.) Melalui Proses Nitrasi dan Hidrolisis Serta Uji Aktivitas Sebagai Antiinflamasi. Jakarta.
Mumpuni, E., L, G. and Nurhidayati, L. 2010. Sintesis p-Metoksisinamoil Urea
dengan Bahan Baku Etil p-Metoksisinamat yang Diisolasi dari Rimpang Kencur (Kaempferia galanga L.)’, Jurnal Farmasi Universitas Pancasila.
Patel , B. R. et al. 2014. Green Efficient Synthesis of Aryl Thioamides Using Ultrasound: A Comparative Study. Journal of Pharmacy And Applied Sciences. 1(1): 29–33.
Pawar, H. S., Wagh, A. S. and Lali, A. M. 2016. Triethylamine: A potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid’, New Journal of Chemistry. Royal Society of Chemistry, 40(6): 4962–4968. doi: 10.1039/c5nj03125g.
Pratiwi et al. 2018. Comparison of esterification and transesterification method in synthesis of octyl p-methoxycinnamate (OPMC) from kaempferia galanga L. rhizome. Rasayan Journal of Chemistry. 11(4): 1618–1623. doi:
10.31788/RJC.2018.1144036.
Sharma, P. 2011. Cinnamic acid derivatives: A new chapter of various pharmacological activities. Journal of Chemical and Pharmaceutical Research. 3(2): 403– 423.
108
Discussion and feedback