AMIDATION CINNAMIC ACID BY DIETHYLAMINE WITH BORIC ACID AS CATALYST USING ULTRASONIC IRRADIATION
on
JURNAL KIMIA (JOURNAL OF CHEMISTRY) 16 (1), JANUARI 2022 DOI: https://doi.org/10.24843/JCHEM.2022.v16.i01.p08
p-ISSN 1907-9850
e-ISSN 2599-2740
AMIDATION CINNAMIC ACID BY DIETHYLAMINE WITH BORIC ACID AS CATALYST USING ULTRASONIC IRRADIATION
Y. Purwaningsih1*, E. S. Hanhadyanaputri2, E. V. Mutiara1, D. I. Dewi1, Y. A. Muthohar1
-
1Program Studi Farmasi, Sekolah Tinggi Ilmu Farmasi Yayasan Pharmasi, Semarang, Jawa Tengah, Indonesia
-
2Program Studi D3 Analis Farmasi dan Makanan, Sekolah Tinggi Ilmu Farmasi Yayasan Pharmasi, Semarang, Jawa Tengah, Indonesia
*Email: y14purwaningsih@gmail.com
ABSTRACT
Amides have important biological activities in medicine and agriculture. Amides can be synthesized in various ways, one of which is the direct reaction of carboxylic acid compounds and amines using boric acid as a catalyst. The purpose of this study was to synthesize N, N-diethyl cinnamamide compounds through direct amidation of cinnamic acid by diethylamine. The catalyst used was 5% mol of boric acid. The reaction process took place in a sonicator bath at 50oC within 40 minutes. The characterization of the compound was carried out by ATR-FTIR and 1H-NMR Spectrometry. The product of the synthesis was a 20,47% yellowish-white solid with a yield of a sapodilla fruit aroma. Identification using ATR-FTIR spectrometry showed several functional groups characteristic for Cinnamamide derivative, namely the C=O amide, C=C, C-N, aliphatic CH groups, monosubstituted benzene which were conjugated by a double bond. Characterization using 1H-NMR showed several chemical shifts for the compound N,N-diethyl cinnamamide. Based on these reason, it could be concluded that the compounds N, N-diethyl cinnamamide can be synthesized with good yields in a short time.
Keywords: amidation, boric acid, cinnamic acid, irradiation ultrasound, and N,N-diethyl cinnamamide.
ABSTRAK
Senyawa amida memiliki aktivitas biologi yang penting dalam bidang pengobatan dan juga pertanian. Amida dapat disintesis dengan berbagai cara, salah satunya adalah dengan reaksi langsung senyawa asam karboksilat dan amina menggunakan katalis asam borat. Tujuan dari penelitian ini adalah untuk mensintesis senyawa N,N-dietil sinamamida melalui amidasi langsung asam sinnamat oleh dietilamina. Katalis yang digunakan adalah asam borat 5% mol. Proses reaksi berlangsung dalam sonikator pada suhu 50oC dalam waktu 40 menit. Karakterisasi senyawa dilakukan dengan spektrometri ATR-FTIR dan 1H-NMR. Hasil sintesis berupa padatan putih kekuningan sebanyak 20,47% dengan aroma buah sawo. Identifikasi menggunakan spektrometri ATR menunjukkan beberapa gugus fungsi yang karakteristik untuk turunan sinamamida yaitu gugus C=O amida, C=C, C-N, CH alifatik, benzena monosubstitusi yang terkonjugasi oleh ikatan rangkap. Karakterisasi dengan 1H-NMR menunjukkan beberapa pergeseran kimia untuk senyawa N,N-dietil sinamamida. Berdasarkan hal-hal tersebut dapat disimpulkan bahwa hasil amidasi asam sinamat oleh dietilamina adalah N,N-dietil sinamamida dengan rendemen yang baik dalam waktu yang singkat.
Kata kunci: amidasi, asam borat, asam sinnamat, iradiasi ultrasonik dan N,N-dietil sinnamamida.
INTRODUCTION
Amides are a group of carbonyl compounds derived from carboxylic acids in which the acyl group is attached to the Nitrogen atom. This compound is widely found in natural products. Amides include an important group of organic compounds
because of the application of these compounds in various fields such as medicine and agricultural chemicals (Pulle, 2020). Several amide derivatives have important biological activities such as antimicrobial, antioxidant (Seelolla, 2014), anticancer (Ernawati & Nurhalimah, 2017 and Fattah et al., 2020) antituberculosis (Permatasari & Ritmaleni,
2019), antidiabetic (Ernawati et al., 2020), antibacterial (Yualanda et al., 2018), and anticonvulsants (Dimmock et al., 2004).
Amide formation is generally carried out by reacting carboxylic acids which are activated by activators such as N, N-disyclohexyl carbodiimide (DCC) with an amine (Ken et al., 2019 and Ernawati & Nurhalimah, 2017). Another method for the synthesis of amides is the use of active carboxylic acid derivatives such as acid chlorides (Lee et al., 2019; Ernawati et al., 2020; Fattah et al., 2020 and Nimse et al., 2015). The use of these materials is increasingly being avoided with the reason that they are not environmentally friendly because they cause hazardous waste (Tang, 2012), so an alternative synthesis method is needed.
One method used is directly react carboxylic acids and amines either with the help of a catalyst (Ahmadi et al., 2018) or not. If a catalyst is not used, the reaction is usually carried out at a high temperature for a long time. The use of a catalyst is an alternative because it can be used at low temperatures with good results (Charville et al., 2011). The alternative catalyst that can be used is boric acid because it is commercially available, environmentally friendly, inexpensive, easy to handle, and stable (Shahrisa et al., 2012).
Boric acid can be used as a catalyst in the synthesis of cinnamamide derivatives (Tang, 2012). Amidation cinnamic acid by amines using boric acid as the catalyst takes a long time by using reflux (Arce et al., 2015 and Dali & Dali, 2017) therefore a method is needed to reduce the reaction time. An alternative method that can be used is sonochemistry with the use of ultrasonic waves. This method can shorten the reaction time with a simple procedure, high yield and environmentally friendly, and also the use of ultrasonic irradiation is a green technology (Ahmadi et al., 2018). Based on this description, this study aimed to synthesize N, N-diethyl cinnamamide compounds through direct amidation of cinnamic acid by diethylamine.
MATERIAL AND METHOD
Materials
All chemicals and solvents required for synthesis and analytical reagent were purchased from Sigma-Aldrich and Merck.
They were cinnamic acid, diethyl amine, boric acid, diethyl ether, and sodium sulfate.
Apparatus
The apparatus used for the research included glassware in the laboratory, melting point were determined with the use of melting point apparatus, sonicator using Cleaning bath ultrasonic Bransonic CPX1800H-E type (45 kHz), rotary evaporator vacuum, analytical balance, the infrared spectra were recorded on Agilent Technologies Cary 630 FTIR, the chemical shift for 1H-NMR by JEOL JNM-ECA500 (500MHz) spectrometer in LPPT UGM.
Procedure for Amidation
This method referred to Mirza-Aghayan et al. (2016) with modification. 10 mmol cinnamic acid was put into an erlenmeyer, then added with 10 mmol diethyl amine and 5% mol boric acid. The mixture was put in a sonicator at 50oC for 40 minutes. The mixture was fractionated with diethyl ether (2x20mL) in a separating funnel and added with 10 mL of distilled water. The organic phase was added with anhydrous sodium sulfate to bind the water and then filtered. The phase of organic that containing amide was evaporated using an evaporator vacuum until a white precipitate was formed. The product was tested for their melting point and characteristic by using ATR-FTIR and 1H-NMR spectrometry.
RESULTS AND DISCUSSION
The direct amidation of cinnamic acid with diethyl amine using boric acid as catalyst was carried out with the aid of ultrasonic waves. The reaction was done in a simple sonicator at 50oC within 40 minutes. The choice of temperature and time was based on the results of orientation with showed the largest yields. The product was a yellowish-white solid with a sapodilla fruit aroma. The mechanism of amidation reaction can be seen in scheme 1.
The process of amidation was done in a simple sonicator with cinnamic acid and diethyl amine as reagents and boric acid as catalyst. Ultrasonic irradiation can accelerate temperature in the medium. The effect of irradiation ultrasonic caused cavitation phenomenon which is a condition for the
formation of bubbles or cavities. The bubble will expand to its maximum size and then burst. The bursting of the bubbles resulted in a shock wave that caused extreme conditions where there was an increase in temperature of up to 5000 K and an increase in pressure of up to 1000 atm (Chatel, 2016). The extreme conditions that occur can cause the breaking of chemical bonds in cinnamic acid and diethyl amine so that it can accelerate the reaction to form N,N-diethyl cinnamamide compounds.
Boric acid reacts with cinnamic acid to produce instead of give anhydride as an acylating agent. The addition of diethyl amine as nucleophile results in N, N-diethyl cinnamamide, and boric acid as a catalyst which are released again (Arce et al., 2015).
O

cinnamic acid
HO OH
B
OH
boric acid
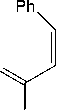

O
different melting points of the N,N-diethyl cinnamamide compound, such as 104oC (Pathan & Agarkar, 2014), 58-60oC (Leggio et al., 2017), and 71-72oC (Dimmock et al., 2004). This different melting point is because the product is not yet pure.
Identification of the product using ATR-FTIR spectroscopy showed some absorption of functional groups characteristic of the compound N,N-diethyl cinnamamide. The functional groups were C = O amide, C = C conjugated benzene, C-N, C = C transsubstituted, monosubstituted benzene, and CH2CH3 groups. The groups absorbed their individual characteristic wavenumber. The C = O amide group was shown in the 1670 cm-1, the C = C group which is conjugated to the benzene ring in the 1629 cm-1. This is also supported by the absorption in the area of 977 cm-1 which indicates the presence of trans substituted C = C. The area at wave number 1204 cm-1 indicates the presence of a C-N group. The presence of the aromatic benzene group was shown by the absorption at 1543 cm-1 and supported by the absorption at 768 cm-1 indicating the presence of monosubstituted benzene. The presence of CH2CH3 alkyl groups was indicated by the absorption at the wavenumber of 1379 cm-1.
Ph
NH(CH2CH3)2 diethylamine
O

N(CH2CH3)2 +
HO OH
B
OH
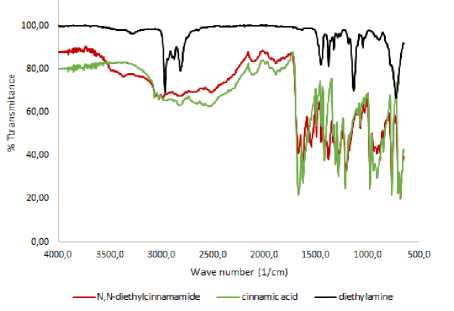
Figure 1. The ATR-FTIR spectrum of N, N-diethyl cinnamamide, cinnamic acid and diethyl amine
N,N-diethylcinnamamide boric acid
Scheme 1. Amidation mechanism of cinnamic acid with diethyl amine using boric acid as catalyst (Charville, 2012)
The average yield of the product was 20.47%. The low yield was caused by the steric hindrance of the diethyl amine (Leggio et al., 2017). The melting point of the product was 114.5 - 117.7oC. Some literature showed
The position of the hydrogen atom in the compound was shown by the spectra of 1H-NMR by observing the chemical shift of each H atom. The chemical shift in the area of 1,370-1399 ppm (t, 6H) indicated the presence of CH3 alkyl groups (positions 11 and 13). δ 3,099 - 3,142 ppm (m, 4H) indicated that there were 4 H atoms in CH2 (C10 and C12)
bonding to CH3. This signal shifts towards the downfield because it binds to the electronegative element, namely –N. The H atom in the C = C bond of the alkene conjugating on the aromatic ring at δ 6.473 ppm (d, 1H) indicated the H position on C2, while the chemical shift of 6.505 ppm (d, 1H) indicated hydrogen at the C3 position. The chemical shift that occurred at δ 7,383-7,768 ppm showed the positions of 5 H atoms in the aromatic ring, which are correlated and equivalent to each other. The chemical shift of the synthesized 1H-NMR spectra showed similarities to a published research done by Leggio et al. (2017), and hence it can be concluded that the synthesized compound was N, N-diethyl cinnamamide.
This ammonium cinnamate salt is stable salt as an intermediate in the reaction between cinnamic acid and amines (Huy & Zoller, 2019). Ammonium cinnamate salt can be hydrolyzed to form amides (Mirza-Aghayan et al., 2016).
O
cinnamic acid

diethylamine
))) H3BO3
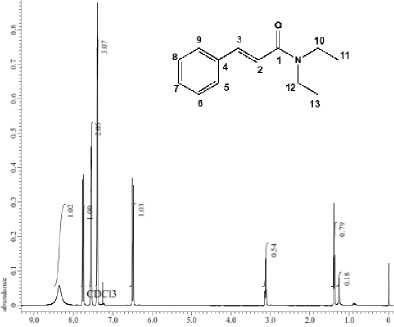
Figure 2. 1H-NMR spectrum of N,N-diethyl cinnamamide
The reaction of cinnamic acid by diethyl amine in this study also formed another compound, namely ammonium salt, this was shown by the chemical shift data of the 1H-NMR spectra at 8.355 ppm. This chemical shift showed that the position of the H atom bonding to N should not appeared for the compound diethyl cinnamamide. The peak that appears at δ 8,355 could occur due to the presence of diethyl ammonium cinnamate salt is formed during the reaction process between carboxylic acids and amines (Charville et al., 2011). This is also supported by the spectrum from infrared which shows the wide absorption of carboxylate ions in the 33002500 cm-1 which is supported by the absorption in the 1577 cm-1 indicating COOstretching and the presence of NH absorption at 3366 cm-1(Coates, 2006).
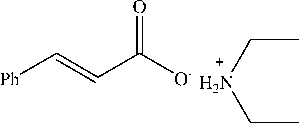
diethylamonium cinnamic salt
Scheme 2. The formation of the diethyl ammonium cinnamate salt (Charville et al., 2011)
CONCLUSION
The direct amidation of cinnamic acid by diethyl amine with boric acid catalyst resulted in the compounds of N, N-diethyl cinnamamide and ammonium cinnamic salt. The reaction could be carried out in a simple procedure and in a short time.
ACKNOWLEDGEMENT
The author was grateful to LPPM Stifar Yayasan Pharmasi Semarang for funding this research and all parties for their help during the research.
REFERENCES
Ahmadi, M., Moradi, L., & Sadeghzadeh, M. 2018. Synthesis of benzamides through direct condensation of carboxylic acids and amines in the presence of diatomite earth@IL/ZrCl4 under ultrasonic
irradiation. Research on Chemical Intermediates. 44(12): 7873–7889.
https://doi.org/10.1007/s11164-018-3592-9
Arce, G., Carrau, G., Bellomo, A., & Gonzalez, D. 2015. Greener Synthesis of an Amide by Direct Reaction of an Acid and Amine under Catalytic Conditions. World Journal of Chemical Education. 3(1): 27–29.
https://doi.org/10.12691/wjce-3-1-4
Charville, H. 2012. Direct Amide Formation Between Carboxylic Acids and Amines: Mechanism and Development of Novel Catalytic Solutions. Durham University.
Charville, H., Jackson, D. A., Hodges, G., Whiting, A., & Wilson, M. R. 2011. The uncatalyzed direct amide formation reaction - Mechanism studies and the key role of carboxylic acid h-bonding. European Journal of Organic Chemistry, 30: 5981–5990.
https://doi.org/10.1002/ejoc.201100714
Chatel, G. 2016. sonochemistry: New Opportunities for Green Chemistry. In World Scientific Publishing Europe Ltd. World Scientific Publishing Europe Ltd.
Coates, J. 2006. Encyclopedia of Analytical Chemistry - Interpretation of Infrared Spectra, A Practical Approach. In R. A. Meyers Ed. Encyclopedia of Analytical Chemistry. John Wiley & Sons Ltd. http://www3.uma.pt/jrodrigues/disciplina s/QINO-II/Teorica/IR.pdf
Dali, N., & Dali, A. 2017. Sintesis N - p-Metilbenzil- p -Kumaramida dari Asam p -Kumarat. Al-Kimia. 5(2): 154–160.
Dimmock, J. R., Gunda, S. G. R., Vashishtha, S. C., Zello, G. A., Nienaber, K. H., Stables, J. P., Allen, T. M., & Santos, C. L. 2004. Anticonvulsants containing the N-(3-aryl-2-propenoyl) amido
pharmacophore. Journal of Enzyme Inhibition and Medicinal Chemistry. 19(4): 303–312.
https://doi.org/10.1080/14756360409162 442
Ernawati, T., Mun’Im, A., Hanafi, M., & Yanuar, A. 2020. Synthesis of cinnamamide derivatives and their α-glucosidase inhibitory activities. Sains Malaysiana. 49(2): 315–322.
https://doi.org/10.17576/jsm-2020-4902-09
Ernawati, T., & Nurhalimah, N. 2017. Sintesis
N -Oktilsinamamid dan Aktivitasnya terhadap Sitotoksik Sel Kanker Leukemia P388 Synthesis N -Octylcinnamamide and Cytotoxicity Activity against P388 Leukemia Cancer Cells. Kimia Valensi. 3(November): 127–133.
Fattah, A., Firdaus, & Soekamto, N. H. 2020. Synthesis of a Cinnamic Acid Derivative and Bioactivity as an Anticancer Based on Result Quantitative Structure Activity Relationship (QSAR) Analysis. Indo. Chim. Acta. 13(1): 23–29.
Huy, P., & Zoller, B. 2019. Boron Lewis Acid Catalysis: How to Synthesize Amides Atom-Efficiently. Nachrichten Aus Der Chemie (Journal of the German Chemical Society GDCh). 67(5): 51–54. https://doi.org/10.1002/nadc.2019408741 8
Ken, M. L., Tagg, T., & Khairul, W. M. 2019. Synthesis and characterisation of N-analineferrocenylamide via carbodiimide coupling. Malaysian Journal of Analytical Sciences. 23(2): 186–196. https://doi.org/10.17576/mjas-2019-2302-02
Lee, J.-S., Zeller, M., Warkad, S. D., & Nimse, S. B. 2019. Synthesis, Characterization, and Crystal Structure of N-(3-nitrophenyl)cinnamamide. Crystals 2019. 9(599): 1–10.
Leggio, A., Bagalà, J., Belsito, E. L., Comandè, A., Greco, M., & Liguori, A. 2017. Formation of amides: One-pot condensation of carboxylic acids and amines mediated by TiCl4. Chemistry Central Journal. 11(1): 1–12.
https://doi.org/10.1186/s13065-017-0318-9
Mirza-Aghayan, M., Tavana, M. M., & Rabah, B. 2016. Sulfonated reduced graphene oxide as a highly efficient catalyst for direct amidation of carboxylic acids with amines using ultrasonic irradiation. Ultrasonics Sonochemistry. 29: 371–379. https://doi.org/10.1016/j.ultsonch.2015.1 0.009
Nimse, S. B., Pal, D., Mazumder, A., & Mazumder, R. 2015. Synthesis of Cinnamanilide Derivatives and Their Antioxidant and Antimicrobial Activity. Journal of Chemistry. 1–6.
https://doi.org/10.1155/2015/208910
Pathan, R. U., & Agarkar, S. V. 2014. SiO 2 Supported Synthesis of N , N
Disubstituted Cinnamamides. Research Journal of Chemical Sciences. 4(5): 56– 58.
Permatasari, D. A. I., & Ritmaleni. 2019. N- ( 2-chlorobenzyl ) formamide , a Novel Synthesized Antituberculosis Evaluation by Microplate Alamar Blue Assay. 170– 173.
Pulle, J. S. 2020. Synthesis Of Amides By Activation Of Carboxylic Acids Using Phosphonitrilic Chloride. Indo American Journal of Pharmaceutical Research. 10(1): 599–604.
https://doi.org/10.1002/chin.200605269
Seelolla, G. 2014. Synthesis, Antimicrobial and Antioxidant Activities of Novel series of Cinnamamide Derivatives having Morpholine Moiety. Medicinal Chemistry. 4(12): 778–783.
https://doi.org/10.4172/2161-0444.1000229
Shahrisa, A., Esmati, S., & Nazari, M. G.
2012. Boric acid as a mild and efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions. Journal of Chemical Sciences. 124(4): 927–931.
https://doi.org/10.1007/s12039-012-0285-6
Tang, P. 2012. Discussion Addendum for: Boric Acid Catalyzed Amide Formation From Carboxylic Acids And Amines: N-Benzyl-4-Phenylbutyramide. Organic Syntheses. 89: 432–437.
https://doi.org/10.15227/orgsyn.089.0432
Yualanda, V. G., Sary, I. P., & Pangaribowo, D. A. 2018. Sintesis dan Uji Aktivitas Antibakteri Senyawa N-Fenil-3,4-Diklorobenzamida (Synthesis and Antibacterial Activity Assay of N-Phenyl-3,4-Dichlorobenzamide). Pustaka Kesehatan. 6(1): 5.
https://doi.org/10.19184/pk.v6i1.6610
65
Discussion and feedback