THE INFLUENCE OF KCL CONCENTRATION ON ENCAPSULATION EFFICIENCY OF YACON-TUBERS EXTRACT’S CHLOROGENIC ACID
on
JURNAL KIMIA (JOURNAL OF CHEMISTRY) 15 (2), JULI 2021 DOI: https://doi.org/10.24843/JCHEM.2021.v15.i02.p10
p-ISSN 1907-9850
e-ISSN 2599-2740
THE INFLUENCE OF KCL CONCENTRATION ON ENCAPSULATION EFFICIENCY OF YACON-TUBERS EXTRACT’S CHLOROGENIC ACID
S. Oktaviyanti dan I G. M. Sanjaya*
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Surabaya, Jawa Timur, Indonesia
*Email: igmasanjaya@unesa.ac.id
ABSTRAK
Enkapsulasi asam klorogenat ekstrak umbi yakon merupakan salah satu cara untuk melindungi senyawa-senyawa aktif menggunakan paduan polimer alam kappa karagenan-locust bean gum dengan penggunaan ion K+ sebagai pengikat matriks melalui metode gelasi ionik. Pada penelitian ini dilakukan pembentukan gel dari kappa karagenan dan locust bean gum dengan bantuan larutan KCl. Tujuan penelitian ini adalah untuk mengetahui pengaruh konsentrasi larutan KCl pada pembentukan gel serta efektifitas enkapsulasinya. Bertambahnya konsentrasi larutan KCl juga membuat gel menjadi lebih kaku. Hasil uji menunjukkan bahwa penggunaan larutan KCl 0,3M dapat menghasilkan nilai efisiensi enkapsulasi sebesar 79,44%. Kemiripan spektra IR antara gel kappa karagenan-locust bean gum, asam klorogenat murni dan asam klorogenat terenkapsulasi menunjukkan bahwa asam klorogenat hanya terperangkap secara fisik dalam matriks kappa karagenan-locust bean gum dan perubahan spektra karakteristik dari kappa karagenan dari 1018 cm-1 menjadi 1023,62 cm-1 pada sampel hasil enkapsulasi menunjukkan adanya interaksi antara gugus OH dari locust bean gum dan kappa karagenan.
Kata kunci: asam klorogenat; enkapsulasi; kappa karagenan, locust bean gum, Yakon
ABSTRACT
The encapsulation of chlorogenic acid of yacon tuber extract is one way to protect the active compounds using naturals polymers kappa carrageenan-locust bean gum with K+ ions as a matrix binder through the ionic gelation method. This process was carried out by forming a gel from kappa carrageenan and locust bean gum with the help of KCl solution. This encapsulation process has purposes to determine the effect of the concentration of KCl solution on gel formation and the effectiveness of its encapsulation. The test results indicated that the use of 0.3M KCl solution resulted in an encapsulation efficiency value of 79.44%. The bonding between the two polymers and the K+ cation was thought to result in a higher encapsulation effectiveness. The similarity of IR spectra among kappa carrageenan-locust bean gum gel, pure chlorogenic acid and encapsulated chlorogenic acid showed that chlorogenic acid was only trapped physically in the kappa carrageenan-locust bean gum matrix and the shift of kappa carrageenan peak from 1018 cm-1 to 1023,62 cm-1 of the encapsulated sample had shown an interaction between OH groups from locust bean gum and kappa carrageenan.
Keywords: chlorogenic acid; encapsulation; kappa carrageenan, locust bean gum, Yacon
INTRODUCTION
Indonesia as a country with fertile natural conditions and high biodiversity is the right place to grow various types of plants, including herbal plants, which are commonly used as ingredients for alternative medicine. Many of these herbs have extraordinary, and often unexpected, benefits including yakon (Smallanthus sonchifolius), although only as an
alternative medium for treatment (Zobel & Talbert, 2007).
The leaves of the yacon plant have been found to contain phenolic compounds, such as chlorogenic, caffeic, and feluric. The leaves also contain protein, lipids, fiber and saccharides, catechonins, terpenes, and flavonoids (Johnson, et al., 2009). The antioxidant activity of the yacon comes from the phenolic content in the yacon plant. L-tryptophan, chlorogenic acid, and other derivatives of caffeic acid were
identified as the main phenolic components of the yacon root. Yacon leaves also have higher levels of phenolic components than in the roots, which the main components are chlorogenic acid and caffeine (Biazon et al., 2016). Meanwhile yacon tubers contain bioactive components of the food fiber soluble prebiotic (SP) (FOS 6-12 g/100 g) and polyphenol antioxidants especially chlorogenic acid (CA 4.85 mg/100 g) (Manrique, et al., 2004) (Yan, et al., 1999).
Yacon leaves are better known as insulin leaves, because yacon leaves contain protein, carbohydrates and fats as well as fructose sugars which cannot be digested by the digestive enzymes but can be fermented by the large intestine (Zobel & Talbert, 2007). Yacon tuber as a natural sweetener could prevent hyperglycemic, hypercholesterolemic, and cancer (Goncales, et al., 2003). Chlorogenic acid content can inhibit glucose metabolism. Chlorogenic acid can interact with other antioxidant compounds in reducing oxidative stress, thereby preventing dysfunction of pancreatic β-langerhans cells. (Johnston et al., 2003). Increased insulin secretion and increased sensitivity to insulin receptors can improve pancreatic β cells in diabetes mellitus patients (Johnson et al., 2009).
Chlorogenic acid is formed by caffeic acid and quinic acid, and its molecular structure has ester bonds, unsaturated double bonds, and polyphenols are the unstable parts of the three. In the process of extracting from plants, chlorogenic acid often occurs by hydrolysis and isomerization of intramolecular ester base migration. Chlorogenic acid can also easily undergo oxidation due to its structural and chemical properties. Additionally, they are unstable in high temperature conditions and they may undergo transesterification reactions during food storage or processing, limiting their food and pharmaceutical applications. In addition, the thermal processing of food products rich in chlorogenic acid can result from the formation of acrylamide. So that this chlorogenic acid requires protection from oxidation, hydrolysis and isomerization after the compound has been extracted from herbal plants.
Encapsulation is a technique for protecting a material that can be solid, liquid or gas using a polymer coating to form a complex layer that covers the core (Kailasapathy, 2002) (Krasaekoopt, et al., 2003). The purpose of the
encapsulation process is to increase the stability and solubility of a compound, to control the release of active compounds, to produce stable solid particles coated with certain coatings and to minimize nutrient loss from a compound (Dubey, et al., 2009). The coatings used in this study were natural polymers in the form of kappa carrageenan and locust bean gum.
Carrageenan is a type of polysaccharide extracted from several species of red seaweed (Rhodophyceae) (Distantina, et al., 2010). Carrageenan itself is a natural additive whose use is widely used in various industries, such as the food and cosmetics industry. Kappa carrageenan is composed of D-galactose-4-sulfate units with β-1,3 bonds and 3,6-anhydro-D-galactose units with α-1,4 bonds. Besides, carrageenan often contains D-galactose-6-sulfate ester and 3,6-anhidro-D-galktose-2-sulfate ester. (Ulfah, 2009). Locust bean gum is a galactomannan similar to guar gum consisting of a β-D-mannopyranose backbone connected (1→4) with branch points from their 6 positions connected to α-D-galactose (i.e., 1→6 -linked α-D-galactopyranose). There is approximately 3.5 (2.8 - 4.9) mannose residue for each galactose residue.
Carrageenan, a natural polysaccharide containing the cross-linking structure of D-galactose-4-sulfate and 3,6- dehydration-D-galactose, is used as a food additive. Due to its glassy nature, carrageenan can be used as an encapsulation coating. The strength of carrageenan gel can be increased by using locust bean gum (Audet et al., 1990) (Audet et al., 1991).
According to Glickman (Glickman, 1983), Kappa carrageenan will form stronger gelation with K+ ions. According to Bayley (1955) -SO3- from κ-carrageenan interacts with K+ through ionic bonds. In particular, the potassium ion interacts simultaneously with two -SO3- anhydrous galactose groups. Kappa-carrageenan in a sol state in the KCl concentration range 0-15 mmol/dm3 (Tecante & Santiago, 2012). In polyelectrolytes, the counterion protects the electrostatic repulsion between the polymer chain and the coil dimensions decrease as the concentration of counterions increases. The counterion in the reaction between kappa carrageenan and KCl solution is the K+ ion.
Therefore, a study was conducted to determine the effect of the concentration of KCl solution on the efficiency of encapsulation using
kappa carrageenan and locust bean gum with a concentration of KCl solution used were (0.1M; 0.2M; 0.3M; 0.4M and 0.5M) and the physical and chemical properties of the optimum encapsulation results.
MATERIAL AND METHOD
Material
The ingredients used in this study were yakon tuber extract, standard chlorogenic acid, kappa karageenan, locust bean gum, saline solution, KCl solution and aquades.
Equipment
The tools used in this research were beaker, measuring cup, dropper pipette, magnetic stirrer, measuring flask, watch glass, stirring rod, volume pipette, HPLC Shimadzu, FTIR thermo scientific nicolet iS10, and Hitachi Flex SEM 1000.
Experiment Procedures
Extraction of Yacon Tubers
Fresh yacon tuber was peeled and cut into 3 cm thickness, soaked in 3% NaCl solution for 3 minutes, then immersed in a solution of lemon juice (1 : 1 v/v) for 10 minutes. Stored in the refrigerator at 5 ℃ during the waiting period. Minimum 3x repetition.
A total of 200 grams of yacon tuber samples were crushed and extracted, then filtered with a buchner funnel. The remaing sample was added with 40 mL distilled water, and the extraction was carried out by ultrasonication for 10 minutes at 40 ℃ and then filtered. The filtrate was freeze-dried for encapsulation.
Encapsulation of yacon tubers extraxt with kappa carrageenan and locust bean gum
Weighing the kappa carrageenan-locust bean gum mixture as much as 3% with a ratio of 1: 1 and dissolving the mixture with 10mL saline solution (0.85% NaCl) in a beaker. The mixture was with a water bath to 80 ℃ using a water bath. 1 mL of thick extract mixed into the kappa carrageenan: locust bean gum mixture. The mixture was stirred until homogeneous while heating in a bath at a temperature of 80 ℃, until the mixture becomes quite liquid. The liquid dropped to form beads using a dropper pipette into a solution of KCl (0.1M, 0.2M, 0.3M, 0.4M, 0.5M) as much as 20 mL. Let the
beads set aside for 35 minutes. Decantate the formed beads and dry them in petri dishes.
Encapsulation Efficiency
The HPLC method was used to test the encapsulation efficiency. The encapsulation results of 1 gram of yakon tuber extract with kappa carrageenan-locust bean gum coating were immersed in 50 mL of phosphate buffer solution pH 7.4. Chlorogenic acid consentration in the results can be determined by HPLC using acetonitrile moving phase at a temperature of 40 ℃ with a flow rate of 1.0 mL / minute and an inject volume of 50μL.
Characterization
For the characterization test, it was determined the spectrum of the encapsulation results of the yakon tuber extract with kappa carrageenan-locust bean gum coating at a wavelength of 400 - 4000 cm-1 and scanned using a SEM instrument.
RESULTS AND DISCUSSION
Encapsulation of yacon tubers extract with kappa carrageenan
This encapsulation process aims to protect chlorogenic acid compounds after the extraction process using natural polymer coatings. A total of 0.15 grams of kappa carrageenan (kappa carrageenan) is white and 0.15 grams of locust bean gum (locust bean gum) is cloudy white in 10 mL of colorless saline solution. The mixture of the two natural polymers is dissolved in a saline solution because kappa carrageenan is easily dissolved in Na+ salt solution at room temperature. Then added 1 ml of yakon tuber extract. After that it is heated at a temperature of 80 ℃. The mixture is taken using a dropper pipette and dropped into a KCl solution to form beads, left for 35 minutes then decanted.
The process of dropping the extract and polymer mixture into the KCl solution is carried out for gelation formation. The gelation of a mixture of kappa carrageenan and locust bean gum occurs due to the presence of intramolecular K+ (Masakuni & Nakamura, 1986). kappa carrageenan interacts with the KCl solution through ionic bonds, K+ ions will interact with SO3- groups (Tetance & Santiago, 2012). This ionic interaction is stronger with K+ ions, whereas with other ions such as Na+ or
Ca2+ the interaction is weak so that it cannot form a strong gel. (Masakuni & Nakamura, 1986). The highest gel strength was obtained in the mixture between kappa carrageenan and locust bean gum at a ratio of 1: 1 with a value of 1276.7 + 223 g/cm2 (Murdinah, et al., 2006). This shows that locust bean gum and kappa carrageenan have synergism in gel strength (FCC, 1997). kappa carrageenan with locust bean gum has better syneresis than using other carrageenan such as iota-carrageenan (Murdinah, et al., 2006).
During the drying process, the kappa carrageenan polymer forms a double helical structure that produces the intersection point of the polymer chain and the cavity between the chains. The locust bean gum chain will fill the cavity between the carpentine kappa chains making the gel more dense and tight (Glickman, 1983).
The addition of the concentration of KCl solution can affect the formation of gelation, the greater the concentration of KCl solution used, the stiffness increases and reduces the strain of the gel so that it is not easily broken (Dunstan, et al., 2001).
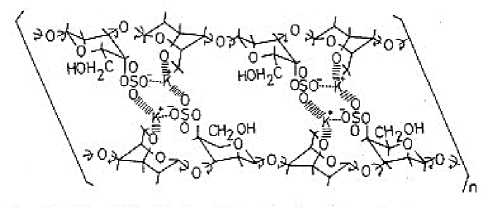
Figure 1. The expected intramolecular form of K+ and kappa carrageenan (Masakuni & Nakamura, 1986).
Encapsulation Efficiency
Coating a chemical compound using kappa carrageenan and locust bean gum can protect the content of these compounds significantly in in vitro conditions (Shi, et al., 2013). In this study, chlorogenic acid compounds will be protected by kappa carrageenan and locust bean gum. The encapsulated beads then tested for the encapsulation efficiency.
This test aims to determine how much of chlorogenic acid that can be coated by kappa carrageenan and locust bean gum. The greater result of the encapsulation efficiency, the greater the chlorogenic acid content that was coated by kappa carrageenan and locust bean gum.
Test of chlorogenic acid levels using the HPLC test showed a peak at 3.2 minutes. A standard curve of chlorogenic acid with concentrations of 5, 10, 15, 20, and 25 ppm produces a curve with a regression value of 0.919 with the equation y = 1.4963x + 0.7922. Here is a standard curve of chlorogenic acid.
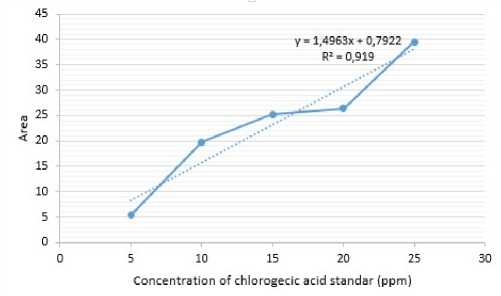
Figure 2. Standard curve of chlorogenic acid
The concentration of KCl solution for the formation of gelation affects the stiffness and density of the gel. The greater the concentration of KCl solution used, the stiffness increases and reduces the gel strain so that it is not easily broken (Dunstan et al., 2001). The protective effect of kappa carrageenan gel can be seen from its intrinsic viscosity. The higher the K+ ion concentration used, the less the intrinsic viscosity value (Tetance & Santiago, 2012). In contrast to gel stiffness, the more K+ ion concentration used, the stiffer the resulting gel will be (Tetance & Santiago, 2012).
According to Shi et al. (Shi, et al. 2013), their research about encapsulation of chlorogenic acid with kappa carrageenan-locust bean gum with Ca2+ cations resulted in an encapsulation efficiency of 60%. The use of K+ cations will help form a stiffer and denser gelation so that it is estimated that it will protect more chlorogenic acid than using Ca2+. Encapsulation with kappa carrageenan-locust bean gum with the K+ cation can produce an encapsulation efficiency of up to 79.44%.
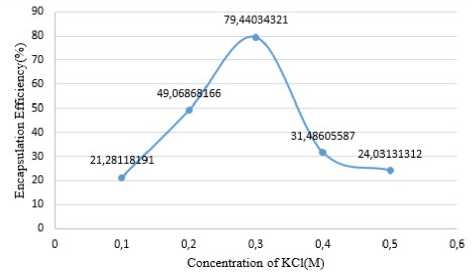
Figure 3. Encapsulation Efficiency Results Cuve
Based on the results of the chlorogenic acid encapsulation efficiency test, it can be observed in Figure 3 that the best results of encapsulation efficiency was obtained at a KCl concentration of 0.3M up to 79.44%. There was an increase in the encapsulation efficiency from using a KCl solution of 0.1M to 0.3M concentration, but there was a decrease in efficiency at 0.4M to 0.5M. This happens because when using a KCl concentration that is lower than 0.3M the gel is weaker so that more chlorogenic acid is released during the process, while using a concentration higher than 0.3M can cause an increase in storage modulus (Stading & Hermansson, 1995). The storage modulus is a measure of how much energy must be put into the sample to distort the sample, in result the encapsulation efficiency with the use of a higher KCl solution is lower (Schaller, 2020). In polymers, the high modulus of storage can cause deformation resistance due to bonding and physical cross-linking (Schaller, 2020).
Characterization
The highest gel strength was obtained in the mixture between kappa carrageenan and locust bean gum at a ratio of 1: 1 with a value of 1276.7 + 223 g/cm2 (Murdinah, et al., 2006). The lowest melting point achieved by the mixture between k-caragen and locust bean gum is 44.0℃ (Murdinah, et al., 2006).
The functional groups of chlorogenic acid encapsulated with kappa carrageenan and locust bean gum polymers can be determined by using the FT-IR test. This test was carried out by comparing the FT-IR results of the kappa carrageenan-locust bean gum encapsulated chlorogenic acid with the FT-IR spectra of kappa carrageenan-locust bean gum gel and pure chlorogenic acid.
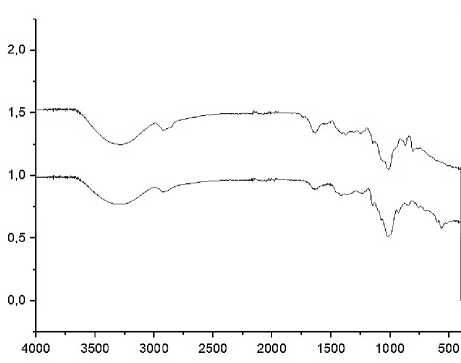
Figure 4. Spektra FTIR of kappa carrageenan (down) and locust bean gum (up)
The characteristics of the kappa carrageenan spectra appear at a wavelength of 1242.19 cm-1 which indicates the presence of a sulfate ester group, at a wavelength of 928.10 cm-1 which indicates the presence of 3.6 anhydrogalactose groups and at a wavelength of 846 cm-1 which indicates the presence of galactose-4-sulfate group which shows the purity of the kappa carrageenan sample. While the locust bean gum spectra have characteristics at wavelengths of 809.98 cm-1 and 870.53 cm-1 which indicate the presence of α-linked D-galactopyranose and β-linked D-mannopyranose groups (Martins, et al., 2012).
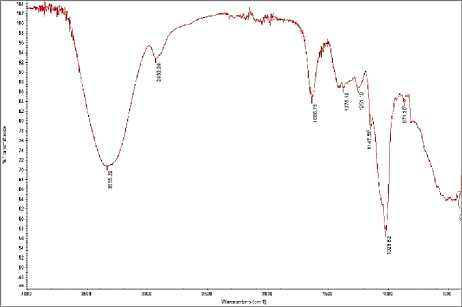
Figure 5. Spectra FTIR of encapsulation results with kappa carrageenan dan locust bean gum
In the FTIR spectra, a peak appears at a wavelength of 3288.37 cm-1 which indicates the O-H group formed by the bond between the polysaccharide and water and at a wavelength of
2925.47 cm-1 which indicates a stretch of the CH group (Cerqueira, et al., 2011).
The chemical interaction between the two polysaccharides can be observed by a shift in the positions of the peaks of their main groups (Wanchoo & Sharma, 2003). This last shift has been attributed to the involvement of C-O in the interaction between the two polysaccharides (Ning, et al., 2007). In the kappa carrageenan spectra, there is a peak at 1018 cm-1 which changes to 1023.62 cm-1 in the encapsulated sample. This is possible because of the CO stretching from the top of the COH group which can be attributed to the interaction (possibly through the hydrogen bridge) of the OH group of locust bean gum with kappa carrageenan structure (Ning et al., 2007). The shift of the other two peaks was also observed in the C-O stretch of the C-O-C group (Ning, et al., 2007) (Pelissari et al., 2009). It can be seen in the locust bean gum spectra that there is a peak at 870.98 cm-1 which changes to 871,30 cm-1 in the encapsulated sample.
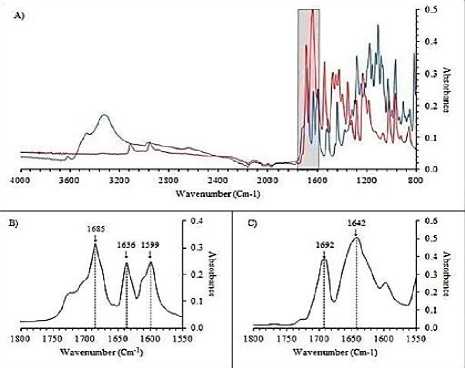
Figure 6. Spectrum of chlorogenic acid standar (Guzman, et al., 2018).
In the spectrum above, at the 1600 - 1800 cm-1 peak of standard chlorogenic acid, 3 clear peaks can be seen, namely the peak of the carbonyl group (C = O) at a wavelength of 1685 cm-1, the ethylene group (C = C) at 1636 cm -1 and a phenyl ring at 1599 cm-1 according to (LIANG, 2016). The characteristic spectra of chlorogenic acid in the encapsulated sample can be seen at a wavelength of 1636.75 cm-1 which indicates the ethylene group (C = C).
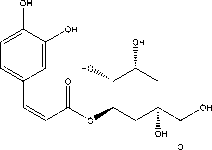
Figure 7. The sructure of chlorogenic acid
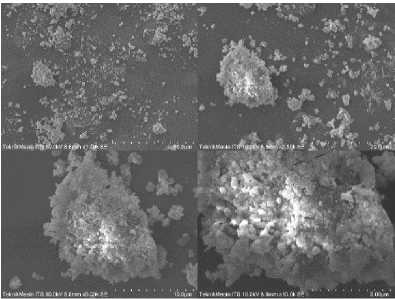
Figure 8. SEM test results of kappa carrageenan dan locust bean gum gel with 1:1 ratio 1000x (a) 2500x (b) 5000x (c) 10000x (d) magnification
In the SEM test results the encapsulation results using kappa carrageenan and locust bean gum above were carried out at a magnification of 1000x. It can be seen that the encapsulated surface consists of fine grains in difference sizes and uneven.
In the SEM results with magnification of 5000X and 10000x, it can be seen that the pores of the encapsulated matrix using kappa carrageenan and locust bean gum. In the picture, it is also seen that the small grains sticking to the surface which are possible for the small grains are compounds in the extract of the yakon tuber (one of which is chlorogenic acid) which are trapped by the kappa carrageenan encapsulants and locust bean gum.
CONCLUSION
Based on the research, it can be concluded that the concentration of KCl solution affects the results of the encapsulation efficiency. The best result of encapsulation efficiency was obtained by using KCl 0.3M solution is 79.44%. At concentrations of KCl solution below 0.3M the encapsulated gel is weaker so that the resulting encapsulation efficiency is lower, while at concentrations higher than 0.3M the gel is more rigidly encapsulated and requires greater energy to
distort the gel resulting low encapsulation efficiency too.
REFERENCES
Audet, P., Paquin, C., and Lacroix, C. 1990. Batch fermentations with a mixed culture of lactic bacteria immobilized separately in k-carrageenan locust bean gum gel beads. Applied Microbiology and Biotechnology. 29.
Audet, P., Paquin, C. and Lacroix, C. 1991. Effect of medium and temperature of storage on viability of LAB immobilized in k-carrageenan-locust bean gum gel beads. Biotechnology Techniques. 32.
Cerqueira, M. A., Souza, B. W. S., Simões, J., Teixeira, J. A., Domingues, M. R. R. M., Coimbra, M. A. 2011. Structural and Termal Characterization of
Galactomannans from Non-Conventional Sources. Carbohydrate Polymers. 83(1): 179-185.
Distantina, S., Fadilah; Rochmadi., Fahrurrozi., Moh., Wiratni. 2010. Proses Ekstraksi Karagenan dari Eucheuma cottonii. Seminar Rekayasa Kimia dan Proses. Solo: Universitas Sebelas Maret.
Dubey, R., Shami, T. C. and Rao, K. U. 2009. Microencapsulation Technology and Applications Vol 59JO. Defence Science Journal.
Dunstan, D. E., Chen, Y., Liao, M L., Salvatore, R., Boger, D V., Prica, M. 2001. Structure and Rheology of the K-Carrageenan/Locust Bean Gum. Food Hydrocolloids, (XV): 475-484.
FCC, 1997. Food Chemical Codex. Institute of Medicine Washington DC.
Glickman, 1983. Food Hydrocolloid vol 1I. Florida: CRC Press Inc Boca Raton.
Goncales, E. J. D., Felicio, M. M., Pinto, M. H., Rossi, C., Medina, M J B., Fernandes, eIC., Simoni. 2003. Inhibition of Aflatoxin Production by Polymnia sonchifolia and its in Vitro Cytotoxicity. Arq. Inst. Biol. 70(2): 159-163.
Guzman, N., Munos, L. and Bahamon, A. 2018. ATR-FTIR For Disc Rimenation of Espresso and Americano Coffee Pods. Research Gate. 550-558.
Johnson, R., Perez-Posa, S E., Sautin, Y Y., Manitius, J., Lozada, L G., Feig, D I. 2009. Hypothesis: Could excessive
fructose intake and uric acid cause type 2 diabetes. Endocr Rev. 30(1): 96-106.
Johnston, K. L., Clifford, M. N., and Morgan, L. M. 2003. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. [Online]
Available at:
https://www.ncbi.nlm.nih.gov/pubmed/14 522730 [Accessed 29 December 2019].
Kailasapathy, K., 2002. Microencapsulation of probiotic bacteria: technology and
potential applications. Curr. Issues Intest. Microbiol. 3(2): 39-48.
Kayaa, A. O. W., Suryanib, A., Santoso, J. and Ruslid, M. S. 2015. The Effect of Gelling Agent Concentration on the Characteristic of Gel Produced From the Mixture of Semirefined Carrageenan and
Glukomannan. International Journal of Sciences: Basic and Applied Research (IJSBAR). 20: 313-324.
Krasaekoopt, W., Bhandari, B. and Deeth, H., 2003. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 13(1): 3-13.
LIANG, N. E. A. 2016. Application of attenuated total reflectance-Fourier transformed infrared (ATR-FTIR) spectroscopy to determine chlorogenic acid isomer profile and antioxidant capacity of coffee beans. Journal of Agricultural and Food Chemistry, 64: 681–689.
Manrique, I., Hermann, M. and Bernet, T., 2004. Yacon- Fact Sheet. Peru: International Potato Center (CIP) Lima.
Martins, J. T., Cerqueira, Miguel A., Bourbon, Ana I., Pinheiro, Ana C., Souza, Bartolomeu W.S., Vicente, António A. 2012. Synergistic Effects Between K-carrageenan and Locust Bean Gum. Food Hydrocolloids. 29: 280-289.
Masakuni, T. and Nakamura, S. 1986. Synergistic Interaction between Kappa-Carrageenan and Locust-bean Gum in Aqueous. Agric. Biol. Chem.
Murdinah, Sinurat, E. and Utomo, B. S. B. 2006. Sifat Fungsional Formula Kappa dan Iota Karaginan Dengan Gum. Jurnal Pascapanen dan Bioteknologi Kelautan dan Perikanan.
Ning, W., Jiugao, Y., Xiaofei, M. and Ying, W. 2007. The Influence of Citric Acid on the Properties of Thermoplastic Starch/Linear Low-density Polyethylene Blends. Carbohydrate Polymers. 67(3), pp. 446453.
Pelissari, F. M., Grossmann, M. V. E., Yamashita, F. and Pineda, E. A. G., 2009. Antimicrobial, Mechanical, and Barrier Properties ff Cassava Starchechitosan Films Incorporated with Oregano Essential Oil. Journal of Agricultural and Food Chemistry. 57: 7499-7504.
Schaller, C. 2020. Libre Texts. [Online] Available at:
https://chem.libretexts.org/Bookshelves/O rganic_Chemistry/Book%3A_Polymer_C hemistry_(Schaller)/04%3A_Polymer_Pr operties/4.08%3A_Storage_and_Loss_M odulus [Accessed 6 October 2020].
Shi, Lu- E., Li, Zhen-Hua., Zhang, Zhi-Liang., Zhang, Ting-Ting., Yu, Wei-Ming. 2013. Encapsulation of Lactobacillus
Bulgaricus in Carrageenan-locust Bean Gum Coated Milk Microspheres With Double Layer Structure. LWT - Food Science and Technology. 54: 147-151.
Stading, M. and Hermansson, A.-M. 1995. Rheology and Microstucture of Mixed K-Carrageenan - Galactomannan Gels. Annual Transactions of the Nordic Rheology Society. 3.
Tecante, A. and Santiago, M. d. C. N. 2012. Rheology. Rijeka: InTech.
Tetance, A. and Santiago, M. d. C. N., 2012. Solution Properties of κ-Carrageenan and Its Interaction with Other Polysaccharides in Aqueous Media.
https://www.intechopen.com/books/rheolo gy/solution-properties-of-k-carrageenan-and-its-interaction-with-other-polysaccharides-in-aqueous-media.
Ulfah, M. 2009. Pemanfaatan Iota Karaginan (Eucheuma spinosum) dan Kappa Karaginan (Kappaphycus alvarezii) Sebagai Sumber Serat Untuk Meningkatkan Kekenyalan Mie Kering. Bogor: Institut Pertanian Bogor.
Wanchoo, R. K. and Sharma, P. K. 2003. Viscometric Study on the Compatibility of Some Water-Soluble Polymerepolymer Mixtures. European Polymer Journal. 39(7): 1481-1490.
Yan, X. J., Suzuki, M., Obnishi-Kameyama, M., Sada, Y., Nakanikshi, T., Nagata, T.
1999. Extraction and Identification of Antioxidants in the Root of Yacon (Smallanthus sonchifolius). Journal of Agricultural and Food Chemistry. 47(11): 4711-4713 .
Zobel, J. and Talbert. 2007. Applied forest tree improvement: wood and tree
improvement. New York: John Willey & Sons, Inc.
202
Discussion and feedback