FLAVONOLS FROM THE LEAVES Lygodium microphyllum (Lygodiaceae)
on
ISSN 1907-9850
FLAVONOLS FROM THE LEAVES Lygodium microphyllum (Lygodiaceae)
Hadi Kuncoro1,2, Kindi Farabi1, Euis Julaeha1, Laode Rijai2, Yoshihito Shiono3 and Unang Supratman1,4,*
1Departement of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Sumedang, West Java, Indonesia
2Laboratory of Pharmaceuticals Research and Development, Tropical Pharmaca, Faculty of Pharmacy, Mulawarman University, Samarinda, 75119, East Kalimantan, Indonesia.
-
3Departement of Bioresources Engineering, Faculty of Agriculture, Yamagata University, Tsuruoka-shi, Yamagata 997-8555, Japan
-
4Central Laboratory of Universitas Padjadjaran, Jatinangor 45363, Sumedang, West Java, Indonesia
*E-mail: unang.supratman@unpad.ac.id
ABSTRACT
Flavonol compounds, quercetin (1) and quercetin-3-O-β-D-glucopyranoside (2) have been isolated from the ethyl acetate extract of Lygodium microphyllum leaves. The chemical structures of flavonol compounds were identified based on spectroscopic data and by comparison of spectral data obtained previously. The discovery of flavonol compounds in Lygodium microphyllum was shown in this study for the first time.
Keywords : Lygodium microphyllum, quercetin, quercetin-3-O-β-D-glucopyranoside, Lygodiaceae.
ABSTRAK
Senyawa flavonol, quercetin (1) dan quercetin-3-O-□ -D-glucopiranosida (2) telah diisolasi dari ekstrak etil asetat daun Lygodium microphyllum. Struktur kimia senyawa flavonol diidentifikasikan berdasarkan data-data spektroskopi dan perbandingan data spektra yang diperoleh sebelumnya. Penemuan senyawa flavonol ini baru dilaporkan pada penelitian ini untuk pertama kali.
Kata kunci : Lygodium microphyllum, quercetin, quercetin-3-O-β-D-glucopiranosida, Lygodiaceae.
INTRODUCTION
Susu A fern has survived since Paleozoic era and can adapt to variety environmental changes (Wallace et al., 1991), thus fern contain a lot of useful secondary metabolites, including flavonoids, steroids, alkaloids, phenols, triterpenoids, various kinds of amino acids and fatty acids (Zeng-fu et al., 2008). One from thousands of ferns species that have interesting pharmacological benefits is Lygodiaceae family. The genus belong to Lygodiaceae family only Lygodium (Guo-gang et al., 2012). Generally, Lygodium genus is a group of ferns that spread and always propagate in other plants. Lygodium genus are different from other kinds of ferns because it
has roots that crawl on the ground rhizomes and fleshy and can only live in the open because they like the sunlight. Some plants from Lygodium genus are invasive and has become a problem in a number of forest areas. It's growing fast and lack of predator makes these plants dominate, displacing wildlife, threatens biodiversity, and enhance the human-animal conflict (Zheng and Xing, 2009). One of the invasive species from Lygodium genus is L. microphyllum. Plants of this genus have a variety of properties that have been widely recognized, thus the utilization of plants from genus is quite expected. Some herbs of Lygodium genus widely used by people one of them as traditional medicine as hepatitis medicine (Zheng and Xing, 2009), back pain, rheumatism
and treatment for kidney stones (Lee et al., 2008), expectorant, scabies, eczema and liver treatment (Upreti et al., 2009), diuretics, antiplasmodial, and treatment for lung and kidney (Upreti et al., 2009), laxative, headache and digestive disorders (Zheng and Xing, 2009). Phytochemical studies on the Lygodium genus had reported to contain a compound with unique structure and had diverse biological activities such as flavonoids (Zhang et al., 2006), phenolic glycoside (Ye et al., 2007), naphthoquinone (Chen et al., 2010), ecdysteroids (Zhu et al., 2009) and phenylpropanoid glycoside (Duan et al., 2012).
L. microphyllum based on ethnobotany and ethnopharmacological information provides many benefits in the field of reproductive health as well as simple patterns make the plant is classified as herbs are easily available so that benefit of these plants is required primarily to determine the chemical constituents of this plant. In our ongoing research to find new biologically active compounds from Indonesia Lygodium plants, we isolated and describe steroid compounds from the leaves of L. microphyllum (Kuncoro et al., 2015). In the further search for biologically active dompounds from the polar fraction, the ethyl acetate extract showed flavonoid constituents. In this paper we decribe isolation and structural elucidation of flavonols from the leaves of L. microphyllum.
MATERIAL AND METHODS
General
Melting point measured on electrothermal melting point apparatus and not corrected. IR spectra were measured on Perkin-Elmer 1760X spectrophotometer, FT-IR on KBr. Mass spectra recorded with a mass spectrometer Water, Qtof HR-MS XEVotm. 1H and 13C NMR spectra are obtained by JEOL NMR 500 MHz, JEOL NMR ECZR 600 MHz used TMS as an internal standard. Chromatographic separation is carried out on silica gel 60 (Merck), octa desyl silane (ODS, Fuji silysia). TLC Plate filled with silica gel GF254 (Merck, 0.25 mm) and detection was obtained with the appearance of 10% H2SO4 in ethanol followed by heating and under ultraviolet-visible light at wavelength of 257 and 364 nm.
Plant Material
The leaves of L. microphyllum was collected from forest areas in Samarinda, East Kalimantan in June 2014. The plant was identified by staff at the Faculty of Forestry, University of Mulawarman, Samarinda and sample specimens stored at the Faculty of Forestry, University of Mulawarman, Samarinda.
Extraction and Isolation
Dried leaves of L. microphyllum (3.54 kg) was extracted with methanol at room temperature. The methanol extract obtained was concentrated at low pressure to produce a concentrated methanol extract (526 g). The concentrated methanol extract was dissolved in water (4: 1) and partitioned successively with n-hexane, ethyl acetate, and n-butanol. Evaporation of the solvents resulted n-hexane (59 g), ethyl acetate (72 g) and n-butanol extract (54 g). The ethyl acetate extract (50 g) was separated using column chromatography with silica gel eluted with n-hexanel-ethyl acetatemethanol as a gradient solvent yield 22 fractions (A01-22). Fraction A10-15 were combined (920 mg) and separated using column chromatography on silica gel eluted with ethyl acetate: methanol (7:3) to obtained 22 fractions (B01-22). Fraction B02-05 were combined (420 mg) and further separated using flash column chromatography on silica gel eluted with a gradient ratio of solvent, ethyl acetate-methanol to obtained 20 fractions (C01-22). Fraction C05 (190 mg) was separated using column chromatography on ODS eluted with a gradient solvent ratio methanol-water to obtain compound 1 (12.5 mg) and 2 (5.4 mg) as a yellowish powder.
Quercetin (2). Yellowish powder; 1H NMR (CD3OD, 500 MHz) see Table 1; 13C NMR (CD3OD, 125 MHz) see Table 1. TOF-MS m/z 303 [M+H]+, calcd. for C15H10O7 m/z 302.
Quercetin-3-O-β-D-glucopyranoside (1). Yellowish powder; m.p 266-268 oC; 1H NMR (CD3OD, 500 MHz) see Table 1; 13C NMR (CD3OD, 125 MHz) see Table 1. TOF-MS m/z 465 [M+H]+, calcd. for C21H20O12 m/z 464.
RESEARCH AND DISCUSSION
The ethyl acetate extract of L. microphyllum leaves was fractionated by a classical column chromatography on silica gel and octa desyl silane adsorbens to yield quercetin (1) and 3-O-β-D-glucopyranoside (2).
Compound 1 was isolated as a yellow amorphous powder. The TOFMS spectrum of 1 gave ion peak at m/z 303 [M+H]+, compatible with the molecular formula C15H10O7. Its UV absorptions in MeOH were consistent with the presence of 3, 5, 7, 3’, 4’-pentahydroxyflavone structure (Wang et al., 2010). In the 1H NMR spectrum of 1, the aromatic region exhibited an ABX system at δH 7.73 (1H, d, J=2.0 Hz, H-2’), 7.62 (1H, dd, J=2.0 and 7.5 Hz, H-6’) and 6.87 (1H, d, J=8.0 Hz, H-5’) due to a 3’,4’-disubstitution of B ring and a typical meta-coupled pattern for H-6 and H-8 protons (6h 6.17 and 6.37, d, J=2.0 Hz). The 13C NMR indicated the presence of 15 atom carbons, the signal at δC 177.3 was attributed to a carbonyl carbon placed at C-4, the other signals were 165.6 (C-7), 162.6 (C-5), 158.4 (C-9), 148.8 (C-4’), 148.1 (C-2), 146.3 (C-3’), 137.3 (C-3), 124.2 (C-1’), 121.8 (C-6’), 116.2 (C-5’), 116.0 (C-2’), 104.6 (C-10), 99.3 (C-6), 94.4 (C-8). The spectral data were compatible with those of quercetin (Fossen et al., 1998; Slimestad et al., 2007).
Compound 2 was obtained as yellowish powder, m.p. 266-268 oC. The molecular formula was established to be C21H20O12 by TOFMS spectra (m/z 465 [M+H]+) together with NMR spectra (Table 1), thus requiring twelve degrees of unsaturation. The UV spectra of 1 showed λ maxima at 353 nm (band I) and 254 nm (band II) characteristic of the quercetin type (Slimestad et al., 2007). The 1H NMR spectrum of 1 showed three aromatic protons signals at δH 7.71 (Ih, d, J=2.0 Hz, H-2’), 6.87 (1H, d, J=8.4 Hz, H-5’) and 7.58 (1H, dd, J=2.0, 8,4 Hz, H-6’) in the form of an ABX spin-system, suggesting a flavonol with 3’,4’-disubstituted B-ring, a pair of meta-coupling proton signals at δH 6.19 (Ih, d, J=2.0, H-6) and 6.38 (1H, d, J=2.0 Hz, H-8) for A-ring. It also showed signals for glucose moiety, it showed
signal at δH 5.23 (Ih, d, J=7.6 Hz, H-1’’) indicated that compound posses β-linked glucose.
The 13C NMR spectra supported this hypothesis and showed 21 signal carbons including a carbonyl signal at δC 179.5 (C-4), five methine sp2, eight sp2 quartenary carbons and six sp3 oxygenated carbons. It revealed chemical shifts at δC 135.6 (C-3), 163.0 (C-5), 165.9 (C-7), 145.9 (C-3’), 149.8 (C-4’) that suggested the 3, 5, 7, 3’, 4’ oxygenated flavone nucleus. It showed significant glucose signals at δC 104.4 (C-1’’), 75.7 (C-2’’), 78.1 (C-3’’), 71.2 (C-4’’), 78.4 (C-5’’) and 62.6 (C-6’’). These functionalities accounted for eight out of the twelve degrees of unsaturation. The remaining four degrees of unsaturation were consistent to a flavonol with a sugar unit (Pereira et al., 2007).
OH
OH
HO
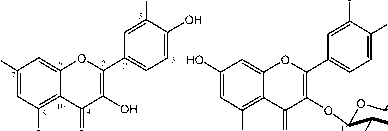
6''
OH
1
OH O HO
2
Figure 1. Chemical structure of compounds 1 and 2
OH
3''
OH OH
The β-configuration for the sugar unit was determined based on the J value information for the anomeric proton (J=7.6 Hz). These data strongly suggest the presence of the sugar unit at C-7 position in the β-configuration. Chemical shifts values at δH 6.44 for H-6 and 6.81 for H-8 are in accordance with values previously reported in the literature for 7-O-glycosylated flavonoids (Pereira et al., 2007). A detailed comparison of NMR data of 1 to those 3-O-β-D-glucopyranoside (Beck et al., 1999; Tang et al., 2000; D’Agostino et al., 1990) showed very similarity, thus compound 2 was identified as a quercetin-3-O-β-glucopyranoside and shown in this species for the first time.
CONCLUSION AND SUGGESTION
ACKNOWNLEDMENT
Conclusion
Two flavonol compounds, quercetin (1) and quercetin-3-O- □ -glucopyranoside (2) were isolated from the leaves of L. microphyllum. The phytocehmical studies of flavonol compounds from this species was shown for first time reported in this study.
Suggestion
The more polar extract from the leaves of L. microphyllum can be further isolated to find novel flavonoid compounds.
Authors gratefully acknowledge to Directorate General of Higher Education, Ministry of Research, Technology and higher education, Indonesia on funds Competitive Research Grant and PKPI Sandwich Like 2015 Scholarship. Our thanks also to Ahmad Darmawan, M.Sc. and Sofa Fajriah, M.Si in Chemical Research Center, Indonesian Institute of Sciences, Serpong, Tangerang
Table 1. NMR data for compounds 1 and 2
|
Position of C |
1 |
2 | ||
|
δH (ΣH, mult. J=Hz) |
δC (mult.) |
δH (ΣH, mult. J=Hz) |
δC (mult.) | |
|
2 |
- |
148.1 (s) |
- |
148.4 |
|
3 |
- |
137.3 (s) |
- |
135.6 |
|
4 |
- |
177.3 (s) |
- |
179.5 |
|
5 |
- |
162.6 (s) |
- |
163.0 |
|
6 |
6.17 d, 2.0) |
99.3 (d) |
6.19 (1H, d, 2.0) |
100.4 |
|
7 |
- |
165.6 (s) |
- |
165.9 |
|
8 |
6.37, d, 2.0) |
94.4 (d) |
6.38 (1H, d, 2.0) |
95.4 |
|
9 |
- |
158.4 (s) |
- |
158.2 |
|
10 |
- |
104.6 (s) |
- |
104.8 |
|
1’ |
- |
124.2 (s) |
- |
124.5 |
|
2’ |
7.73 (1H, d, 2.0) |
116.0 (d) |
7.71 (1H, d, 2.0) |
116.5 |
|
3’ |
- |
146.3 (s) |
- |
145.9 |
|
4’ |
- |
148.8 (s) |
- |
149.8 |
|
5’ |
6.87 (1H, d, 8.0) |
116.2 (d) |
6.87 (1H, d, 8.4) |
116.5 |
|
6’ |
7.62 (1H, dd, 2.0, 7.5) |
121.8 (d) |
7.58 (1H, dd, 2.0, 8,4) |
120.8 |
|
1’’ |
5.23 ((1H, d, 7.6) |
104.4 | ||
|
2’’ |
3.48 (1H, t, 9.2) |
75.7 | ||
|
3’’ |
3.35 (1H, t, 8.8) |
78.1 | ||
|
4’’ |
3.43 (1H, t, 9.6) |
71.2 | ||
|
5’’ |
3.24 (1H, m) |
78.4 | ||
|
6’’ |
3.73 (1H, dd, 11.6, 2.0) 3.56 (1H, dd, 11.6, 5.2) |
62.6 | ||
REFERENCES
Chen, L., Zhang, G., He, J., Jin Guan, J., Pan, C., Mi, W., and Wang, Q. 2010. New naphthoquinone from the root of
Lygodium japonicum (Thunb.) Sw. Journal of Natural Medicine 64, 114– 116.
Beck, M.A., Haberlein, H. 1999. Flavonol glycosides from Eschscholtzia
california. Phytochemistry, 50, 329
332.
Duan, Y., Dai, He, Y. R., Kurihara, H., Li, Y., and Yao, X. 2012. A new phenylpropanoid glucoside from the aerial parts of Lygodium japonicum. Journal of Asian Natural Products Research, 14 (3), 286–292.
D”Agostino, M., De Simone, F., Dini, A., Ramundo, E., Zollo, F., 1990. Flavonol glycosides from Eupatorium tinifolium. Phytochemistry, 29, 353-354.
Fossen, T., Pedersen, A.T., Andersen, O.M., 1998. Flavonoids from red onion (Allium cepa). Phytochemistry, 47, 281-285.
Guo-Gang, Z., Ying-Cui, H., Hong-Xia, L., Lin-Xia, Z., and Li-Juan, C. 2012. The Research of Lygodium. Intechopen. Shanghai.
Kuncoro, H., Farabi, K., Julaeha. E., Rijai. L., and Supratman, U., 2015,
Stigmast-5(6)-en-3β-ol dari herba
tumbuhan krokot (Lygodium
microphyllum), Jurnal Kimia Valensi, 1(1), 50-54.
Lee, S., Xiao, C., and Pei, S. 2008. Ethnobotanical survey of medicinal plants at periodic markets of Honghe Prefecture in Yunnan Province, China. Journal of Ethnopharmacology. 117, 362–377.
Pereira, A.P., Pereira, I.C., Marcelino, F., Valentao, P., Andrade, P.B., Seabra, R., Estevinho, L., Bento, L., Pereira, J.A. 2007. Phenolic compounds and antimicrobial activity of olive (Olea europea L. cv Cobrancosa) leaves. Molecules, 12, 1153-1162.
Slimestad, R., Andersen, O.M., Francis, G.W., Marston, A, A., Hostettmann. 1995.
Syringetin 3-O-(6″-acetyl)-β-
glucopyranoside and other flavonols from needles of norway spruce, Picea abies. Phytochemistry, 40, 1537-1542.
Tang, Y., Wang, Y., Lou, F., Li, Y., Wang, J., 2000. Flavonol glycosides from leaves of Ginkgo biloba. Acta Pharm. Sin. 35, 363-366.
Upreti, K., Jalal, J. S., Tewari, L. M., Joshi, G.C., Pangtey, Y. P. S., and Tewari, G. 2009. Ethnomedicinal uses of Pteridophytes of Kumaun Himalaya, Uttarakhand, India. Journal of American Science. 5, 167–170.
Wallace, R. A., Sander, G. P., and Ferl, R. J. 1991. Biology: The Science of Life. Harper Collins. New York.
Wang, J., Gao, H., Zhao, J., Wang, Q., Zhou, L., Han, J., Yu, Z., Yang, F.,. 2010. Preparative separation of phenolic compounds from Halimodendran halodendron by high-speed countercurrent chromatography. Molecules, 15, 5998-6007.
Ye, W., Fan, C., Zhang, L., Yin, Z., and Zhao, S. 2007. A new phenolic glycoside from the roots of Lygodium japonicum. Fitoterapia. 78, 600–601.
Zhang, L., Yin, Z., and Ye, W. 2006. Flavonoids from Lygodium japonicum. Biochemical Systematics and Ecology. 34, 885-886.
Zeng-fu, L. I., Huil, H., Hang-yi, Z., and Jun-chen, Z. 2008. Review on the extraction of flavonoids from fern. Journal of San University. 25, 22.
Zheng, X. L. and Xing, F. W. 2009. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. Journal of
Ethnopharmacology. 124, 197–210.
Zhu, L., Zhang, G., Chen, L., Wang, S., Li, P., Li, L. 2009. A new ecdysteroside from Lygodium japonicum (Thunb.) Sw. Journal of Natural Medicinal. 63, 215– 219.
14
Discussion and feedback