PHOSPHATASE ACTIVITY AND PHOSPHATE SOLUBILITY BY PHOSPHATE SOLUBILIZING RHIZOBACTERIA IN VOLCANIC SOILS OF PANCASARI, BALI
on
ORIGINAL RESEARCH ARTICLE
Phosphatase Activity and Phosphate Solubility by Phosphate Solubilizing Rhizobacteria in Volcanic Soils of Pancasari, Bali
Ketut Dharma Susila1*, I Made Sudana1, Ni Putu Ristiati 2, and I Made Adnyana1
1Faculty of Agriculture, Udayana University, Bali 2University of Ganesha Education
* Corresponding author : soesila99@yahoo.co.id
Received : 21st April 2016 | Accepted : 24th August 2016
ABSTRACT
Phosphatase in the soil was found as extracellular enzymes produced by soil microorganisms both in acidic or alkaline conditions. Phosphatase is an enzyme complex that plays an important role in deciding soil-bound phosphate bond organic compounds to form orthophosphate which is available to the plant. Mineralization of organic forms into a inorganic-P determined by the ability of these bacteria to produce phosphatase. Therefore, observations of phosphatase activities are important to know how intensely P mineralization process takes place in the soil. Phosphate solubilizing rhizobacteria (PSR) has been widely known to affect mobilization of insoluble inorganic phosphates become available to plants. Although phosphate solubilizing rhizobacteria is widely available in the soil, their activity is usually not strong enough to compete with other bacteria commonly located in the root zone (rhizosphere). That is why, screening of phosphatase enzyme activity and various phosphate solubilizing ability to obtain better strains of bacteria are still needed. This study was conducted to determine the ability of phosphate solubilizing rhizobacteria, its potential as a biofertilizer inoculant and characterize its capacity to dissolve P-insoluble inorganic and produce phosphatase enzymes in the growing medium. Potential phosphate solubilizing rhizobacteria was tested for their ability in vitro using Pikovskaya media containing insoluble forms of inorganic phosphate as a source P. The results of this study indicate that there are some isolates phosphate solubilizing rhizobacteria that are capable of dissolving the insoluble inorganic-P with different abilities. The highest potential successively displayed by isolates TbPP-4.1; BdPP-2.1; and SBPP-1.3 that significantly has the same ability to soluble inorganic phosphate in the soil. The isolates BdPP-2.1 and TbPP-4.1 are significantly higher than the other isolates for producing phosphatase. Both isolates TbPP-4.1 and BdPP-2.1 have the same pontenty as a biofertilizer based on its capacity to mineralize forms of soil organic phosphorus by phosphatase activity.
Key words : soil phosphorus, solubility, phosphatase, mineralization, inorganic insoluble-P
INTRODUCTION
Phosphorus is the second most essential nutrient for plants, after nitrogen and is a source of non-renewable nutrient lately received
much attention. Average total phosphorus in soil range from 0.05% (w / w), but only 0.1% can be utilized by plant (Zhu et al., 2011). Most of inorganic phosphorus available were
ISSN ONLINE: 9 772303 337 008
given to the soil through chemical fertilizers, quickly will be bound by the soil immediately after application and become unavailable to the plants. This means that the soil will contain a lot of phosphorus reserves, but availability is low due to low diffusion process and the high power to phosphorus soil fixation. As reported by Islam et al. (2006) that the availability of phosphorus in the soil is usually low both at low pH values and high pH under the conditions of the highlands. Its means that phosphorus could be a major limiting factor for plant growth. Therefore, giving back P in the forms of soil phosphorus bound and insoluble become an important aspect in the effort to increase the availability of phosphorus in the soil.
Broadly, the soil microorganisms involved in many biological processes includes transformation of soil phosphorus. Phosphate Solubilizing Rhizobacteria (PSR) has been able to mobilize / solubilize phosphate-inorganic insoluble becomes phosphorus available to plants. They are effectively reportedly capable of releasing phosphorus from organic forms and inorganic in the soil through the
process of mineralization and solubilization.
In the rhizosphere, some bacteria will move insoluble phosphate to produce organic acids to solubilize forms P-inorganic and produce phosphatase enzymes to mineralize forms of soil P-organics. Phosphatase will catalyze the hydrolysis reaction from organic-phosphomonoester become P-inorganic usable for plants. However, the dissolution process P is a complex phenomenon and a lot depends on environmental factors, such as soil nutrient, climate, physiology and growth conditions in the growing medium used. PSR plays an important role in nutrient of plants through increased availability of soil P to plants, and highly prospective use them as biofertilizers for agricultural crops in the future.
Despite the presence of phosphate solubilizing rhizobacteria (PSR) are mostly in the rhizosphere of plants, their activities are often not strong enough to compete with most other bacteria that already exist in the soil (Jain et al., 2012). The ability to produce organic acids in the process of solubilization P and phosphatase activity of PSR is one determinant of
the quality of isolates PSR as a biofertilizer. Therefore, the selection ability of dissolving P-inorganic soil and phosphatase enzyme activity to mineralize soil P-organics are still needed in order to obtain an effective strain isolates.
Pancasari village is located at Bedugul region that is one of agriculture farms to supporting horticultural products in Bali. Its have the volcanic soils with intensively agricultural activities. In their agricultural practices, P is supplied to plants through synthetic phosphatic fertilizers, which indeed is expensive and environment disruptive. The use of phosphate solubilizing rhozobacteria as an alternative to chemically synthesized phosphatic fertilizer is therefore an urgent requirement in crop production system.
The purpose of this study is screening PSR potential as a biofertilizer and measure its ability to dissolve P and produce phosphatase on in vitro condition.
MATERIALS AND METHODS
Isolation and Selection Qualitative Capabilities of Bacteria
About 100g a composite rhizosphere soil sample from some kinds of plants carried to the laboratory
in an ice box and isolation. The soil sampling procedure for soil biological analysis and protocols are described by Saraswati et al., (2007). A total of 10 g rhizosphere soil sample was mixed with 90 ml of sterile physiological saline solution (0.85% NaCl) and shaken for 120 minutes. It was then dilute from 10-1 till 10-5dilution series, the suspension is then distributed / inoculated into media selection in a petri dish which has been filled with Pikovskaya solid media containing 10 g of glucose, 5 g of Ca3 (PO4) 2, 5 g MgCl2 • 6H2O, 0, 25 g, MgSO4 • 7H2O, 0.2 g KCl, and 0.1 g (NH4) 2SO4 in 1 L of distilled water (Nautiyal, 1999). The pH was adjusted to 7 with HCl before autoclaving. Furthermore, media was incubated for 7 days at room temperature (28-+2°C).
Bacterial colonies surrounded by a halo on Pikovskaya media were indicate to be phosphate solubilizers. Colonies with a big size of clear zone (halo) were considered as potentially phosphate solubilizing rhizobacteria (Chaiharn and Lumyong, 2009). There are 12 isolates PSR have been isolated based on their ability to form a clear zone in that petri dish.
ISSN ONLINE: 9 772303 337 008
Measurement of Quantitative Phosphate Solubilizing in Vitro Conditions
Based on the appearance of the ability of the formation of a clear zone, there are 12 phosphate solubilizing rhizobacteria (PSR) were elected, then set its ability to solubilize the phosphate in the media culture in vitro. At first, they were grown or rejuvenated in advance erlenmeyer with a liquid Pikovskaya media with Ca3(P04)2 as a sources P, then put into shaker and incubated for 3 days at room temperature 30 ° C . Each isolate to be measured was added with 10 mL extractors Bray (extracts 25% HCl), shaken and allowed to stand for 5 hours. It was then centrifuged with a rotation speed of 10,000 rpm for 15 minutes and the supernatant of each sample was taken 2 mL. Isolate was incorporated into a clean and sterile test tube. Further along the standard sequence of each isolate PSR were added 10 ml phosphate dye reagents, shake until homogeneous and leave for 30 minutes. Absorbance of the solution was measured with a spectrophotometer at a wavelength of 693 nm. P205 assay quantitatively dissolved is calculated based on the
standard curve equation standard solution 100 ppm PO4 Titrisol according to Technical Guidelines Soil Research Center, Bogor (2005).
Measuring Phosphatase Activity
Determination of activity phosphatase enzyme was started by making a standard curve of p-nitrophenol (p-NP) and then stored at temperature 4o C.
Phosphatase enzymes measurements are performed according to procedure Eivazi and Tabatabai (1977). A total of 10 ml PSR cell cultures was grown for 3 days at room temperature in a liquid Pikovskaya medium with Ca3(PO4)2 as a source of P, centrifuged at 10,000 rpm for 15 minutes to separate the bacteria colonies in the medium.
Supernatant was then transferred into a 1 ml clean and sterile test tube, then added with 4 ml Modified Universal Buffer (MUB), and 1 ml of p-nitro phenyl phosphate (p-NPP,15mM), incubated for 60 min in a water bath at 37ºC temperature. After 60 minutes incubation, 4 ml of 0.5 N NaOH was added to stop the reaction. The solution will change to yellow color, indicating that the bacterium
produces phosphatase enzyme
(Saraswati et al., 2007).
Samples and control OD value was measured at a wavelength of 400 nm using a Hitachi 150-20 type Spectrophotometer. OD values of samples and controls are converted into phosphatase enzyme activity based on the equation of p-nitrophenol standard solution that was created earlier. Analysis of phosphatase enzymes activity was conducted at the Center for Research and Development of Biotechnology and Genetic Resources, Bogor.
RESULTS AND DISCUSSION Qualitative Solubilization Capability of PSR
Phosphate Solubilizing
Rhizobacteria (PSR) capability in solubilizing phosphate qualitatively evaluated based on their ability to form a clear zone (halo) in order to petri
dishes containing solid media Pikovskaya. Soil microorganisms that can be grown on solid media will dissolve Pikovskaya P marked by the colored translucent zone. This indicates 2 that there dissolving Ca3 (PO4)2
contained in the Pikovskaya media. However, not all soil microorganisms can form a clear zone.
There are a variety size of the clear zone is formed, this indicates that a variety of bacterium isolated qualitatively phosphate solubilizing capacity varies. The diversity of these capabilities can be seen from the variation of the size of the clear zone is formed, as shown in Figure 1. Furthermore, after purification to obtain single isolates, 12 isolates elected PSR tested for their ability to quantitatively dissolve phosphate. PSR single isolates purification results visualized in Figure 2.
Figure 1. Some colonies PSR Surrounded Halo

Figure 2. Purification of Some Single Isolates PSR
ISSN ONLINE: 9 772303 337 008
Dissolution of Inorganic Phosphate in Vitro Condition
The existence of phosphate in the soil naturally present in organic and inorganic form. The second form is a form of phosphate insoluble or slightly soluble, so its availability for plants and soil microorganisms are very limited. Inorganic phosphate minerals in general are bound as AlPO4.2H2O (variscite) and FePO4.2H2O (strengite) on acid soils and as Ca3(PO4)2 (tricalcium phosphate) on alkaline soils. Organic acids play an important role in the dissolution of phosphate because the organic acids are relatively rich in functional groups carboxyl (COO-) and hydroxyl (-O-) negatively charged making it possible to form complex compounds with the ions (cations) of metals called chelate (Wagner and Wolf, 1998).Organic acids chelate clicking Al, Fe or Ca, resulting in phosphate insolubled AlPO4.2H2O, FePO4.2H2O, or Ca3(PO4)2 thereby increasing-soluble phosphate levels in the soil. This situation will increase the availability of phosphate in the soil solution.
The principal mechanism for mineral phosphate solubilization is the production of organic acids, and acid
phosphatases play a major role in the mineralization of organic phosphorus in soil. It is generally accepted that the major mechanism of mineral phosphate solubilization is the action of organic acids synthesized by soil microorganisms (Rodriguez and Fraga, 1999).
There are 12 isolates tested PSR selected for its ability to quantitatively dissolve phosphate. Results of quantitative determination of the dissolution of phosphate by the PSR isolates are presented in Table 1. Furthermore, the average capacity of dissolving each isolate PSR is presented in Figure 3.
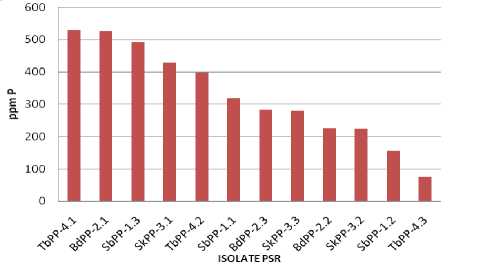
Figure 3. Inorganic Phosphate Solubilizing by PSR Isolates
The results of this study showed that there are some isolates of phosphate solubilizing rhizobacteria (PSR) be able to solubilize insoluble phosphate with varying capability. According to Duncan’s multiple
comparison test (Table 1), isolates TbPP-4.1; BdPP-2.1; and SbPP-1.3 have the same ability to dissolve inorganic phosphate. All three isolates have the ability to very significant compared to other ones. The big differences in ability for each other isolates, possibly due to differences in the number and kinds of organic acids produced (Setiawati et al., 2014; Deubel and Merbach, 2005). The efficiency of solubilization, however, depends on the kind of organic acids released into the medium and their concentration (Nautiyal, 1999).
Table 1. Solubilizing P by PSR Isolates
|
No. |
Isolate Code |
Soluble P Average | |
|
( ppm P ) | |||
|
1 |
TbPP-4.1 |
528.80 a | |
|
2 |
BdPP-2.1 |
525.33 a | |
|
3 |
SbPP-1.3 |
490.33 |
a b |
|
4 |
SkPP-3.1 |
428.83 |
b c |
|
5 |
TbPP-4.2 |
397.38 |
c d |
|
6 |
SbPP-1.1 |
318.62 |
d e |
|
7 |
BdPP-2.3 |
283.24 |
e f |
|
8 |
SkPP-3.3 |
279.98 |
e f |
|
9 |
BdPP-2.2 |
225.93 |
f g |
|
10 |
SkPP 3.2 |
223.44 |
f g |
|
11 |
SbPP 1.2 |
155.40 |
g h |
|
12 |
TbPP 4.3 |
74.61 h | |
|
The |
average value |
in the same column | |
marked with the same letter are not
significantly different according to
Duncan's Multiple Range Test at 5% level
Furthermore, the quality of the acid is more important for P-solubilization than the total amount of
acids produced by phosphate solubilizing (PS) organisms (Scervino et al. 2010a, b). Additionally, the simultaneous production of different organic acids by the PSR strains may contribute to the greater potential for solubilization of insoluble inorganic phosphates (Marra et al. 2012).
According to Sagoe et al. (1988) said, phosphorus solubilizing activity is determined by the ability of microbes to release metabolites such as organic acids, which through their hydroxyl and carboxyl groups chelate the cation bound to phosphate, the latter being converted to soluble forms. Microorganisms through secretion of different types of organic acids e.g. carboxylic acid (Deubel and Merbach, 2005) and rhizospheric pH lowering mechanisms (He and Zhu, 1988) dissociate the bound forms of phosphate like Ca3(PO4)2.
Organic acids produced by PSR solubilize inorganic insoluble phosphates by lowering the pH, chelation of cations and competing with phosphate for adsorption sites in the soil (Nahas, 1996). Inorganic acids e.g. hydrochloric acid can also solubilize phosphate but they are less
ISSN ONLINE: 9 772303 337 008
effective compared to organic acids at the same pH (Kim et al., 1997).
Mineralization of P-organic by Phosphatase Activity
P-organic mineralization determined by into inorganic P- the ability of these bacteria to produce enzymes, such as the phosphatase enzyme. Phosphatase is an enzyme complex that plays a role important in deciding soil-bound phosphate bond organic compounds to form orthophosphate which is available to the plant. In the soils, complex phosphatase consisting of phosphomonoesterase enzyme, phosphodiesterase, and phosphotriesterase (Alef and Nannipieri, 1995).
According to a series of chemical composition, soil organic Precognized in the form of the composition: (a) carbon-phosphorus (chain C-P) called phosphonates; (B) the composition of phosphorus-oxygen-phosphorus (P chain-O-P) which is referred to as a polyphosphate; and (c) the structure of the carbon-oxygen-phosphorus (chain C-O-P) is called a phosphate ester. Most P-dominated soil organic chemical composition esterphosphate groups,
namely monoesterphosphate; diesterphosphates and three-esterphosphates (Suliasih and Rachmansyah, 2013). That is why Tabatabai (1982) suggest that for P-organic mostly in the form of phosphoric ester, an observation phosphomono-esterase enzyme activity or phosphodiesterse enough to represent to determine the phosphatase enzyme activity in the soil (Eivazi and Tabatabai, 1977).
Determination of phosphatase in this study, based solely on the measurement of phosphatase activity produced by the bacteria, which is 12 isolates PSR. Phosphatase measured at this observation is tested through the ability of each PSR in synthesising organic phosphate p-nitrophenol phosphate into p-nitrophenyl. The p-nitrophenol compound formed by the activity of the enzyme then stain with a solution of sodium hydroxide, the solution became yellow. The more intense the yellow color is formed, indicate the higher the phosphatase enzyme produced (Saraswati et al., 2007).
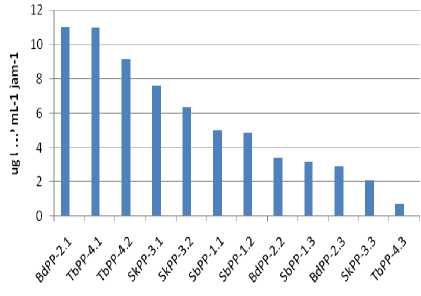
The measurement results phosphatase activity by 12 isolates PSR
and significant differences were determined by using Duncan's Multiple
Comparison Test presented in Table 2,
then the average of phosphatase activity to mineralize organic-P illustrated in Figure 4.
Table 2. Fosfatase Activity of PSR Isolates
|
No. |
Isolat Code |
Phosphatase Activity ( μg PNP mL-1 h-1 ) |
|
Average | ||
|
1 |
BdPP-2.1 |
11,039 a |
|
2 |
TbPP-4.1 |
10,967 a |
|
3 |
TbPP-4.2 |
9,137 b |
|
4 |
SkPP-3.1 |
7,601 c |
|
5 |
SkPP-3.2 |
6,351 d |
|
6 |
SbPP-1.1 |
5,010 e |
|
7 |
SbPP-1.2 |
4,875 e |
|
8 |
BdPP-2.2 |
3,369 f |
|
9 |
SbPP-1.3 |
3,170 f |
|
10 |
BdPP 2.3 |
2,910 fg |
|
11 |
SkPP 3.3 |
2,081 g |
|
12 |
TbPP 4.3 |
0.731 h |
The average value in the same column marked with the same letter are not significantly different according Duncan's Multiple Range Test at 5% level.
PNP
ISOLAT RPF
Figure 4. Fosfatase Activity of PSR
Isolates
The experimental results showed that the content phosphatase on the medium phosphate by producing PSR isolates are significantly different for each other. The isolate BdPP-2.1 and TbPP-4.1 are significantly higher than the other ones for producing phosphatase (Table 2). Isolates BdPP-2.1; TbPP-4.1; TbPP-4.2; SKPP
3.1; SKPP-3.2 and-1.1 SBPP capable of producing phosphatase enzyme as much as 11.039; 10.967; 9.137; 7.601; 6.351 and 5.010 µg mL-1 h-1 respectively and all of them are potentially as a biological fertilizer inoculants. The PSR strains exhibit organic P mineralizing abilities ranging between 0.73-11,039 µg PNP mL-1 h-1 , but Tao et al.(2008) reported that an organic P mineralizing abilities ranging between 8–18 µg P mL-1 h-1 . The differences are possible because of the ability to produce phosphatase is highly dependent on the type isolates PSR, biomass, energy source and growth condition in the composition of the media (Nautiyal, 1999; Khan et al., 2014). Observations phosphatase in relation to the availability of carbon, nitrogen and soil acidity levels showed a positive correlation as the result of research carried out by Kramer and
ISSN ONLINE: 9 772303 337 008
Green (2000). Of the phenomenon can be assumed that the phosphatase activity is sensitive to changes in their environment.
REFERENCES
Alef, K. and P. Nannipieri. 1995. Methods in Applied Soil Microbiology and Biochemistry. Academic Press. Harcout Brace & Company Publishers. London, San Diego, New York, Boston, Sydney, Tokyo, Toronto. 575 p.
Chaiharn, M. and S. Lumyong. 2009.
Phosphate Solubilization
Potential and Stress Tolerance of Rhizobacteria from Rice Soil in Nothern Thailand. World J. Microbiol.Biotechnol. 25: 305 -314.
Deubel, A. and W. Merbach. 2005. Influence of Microorganisms on Phosphorus Bioavailability in Soils. In: F. Buscot and A. Varma (eds.), Microorganisms in Soils: Roles in Genesis and Functions. Springer-Verlag, Berlin
Heidelberg, Germany. p. 62.
Eivazi F, and Tabatabai, M.A. 1977. Phosphatases in Soil. Soil Biol Biochem 9: 167-172.
Jain R, Saxena J, Sharma V. 2012. Solubilization of inorganic phosphates by Aspergillus awamori S19 isolated from rhizosphere soil of a semi-arid region. Ann Microbiol 62:725– 735.
Khan M.S., A. Zaidi, and E. Ahmad. 2014. Phosphate Solubilizing
Mocroorganisms.
DOI.10.1007/978-3319-08216-5 c) Springer International
Publishing, Switzerland.
Kim KY, Jordan D, Krishnan HB (1997) Rahnella aquatilis,
bacterium isolated from soybean rhizosphere, can solubilize
hydroxyapatite. FEMS Microbiol Lett. 153:273–277.
Nahas, E., 1996. Factors determining rock phosphate solubilization by microganisms isolated from soil. World J. Microbiol. Biotech., 12: 567–72.
Nautiyal, C. Shekhar. 1999. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Letter 170, 265-270.
Rodríguez, H. and R. Fraga. 1999. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 17:319-339.
Sagoe, C. I., T. Ando, K. Kouno and T. Nagaoka. 1998. Relative
importance of protons and solution calcium concentration in phosphate rock dissolution by organic acids. Soil Sci. Plant Nutr. 44:617-625.
Saraswati, R., Edi Husen dan RDM Simanungkalit. 2007. Soil Biological Analysis Methods. Agricultural Development and Research Department. Bogor. 291 p.
Scervino JM, Mesa MP, Mo ´nica ID, Recchi M, Moreno NS, Godeas A (2010a) Soil fungal isolates
produce
48 • ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM
Discussion and feedback