MORPHOLOGICAL CHARACTERS AND GENETIC VARIABILITY OF CHAMPACA IN BALI
on
ORIGINAL RESEARCH ARTICLE
MORPHOLOGICAL CHARACTERS AND GENETIC VARIABILITY OF CHAMPACA IN BALI
I Made Sukewijaya1*, Made Sudiana Mahendra1, I Nyoman Rai1, and Gede Rai Maya Temaja2
1Laboratory of Agronomy and Horticulture Udayana University, Bali 2Laboratory of Pathology, Udayana University, Bali
* Corresponding author : imsukewijaya@yahoo.com
Received : 20th June 2016 | Accepted : 23rd August 2016
ABSTRACT
Bali community utilize champaca flower for offering materials and worship, besides beauty salon purposes, the SPA aromatic ingredients, essential oils, perfumes, cosmetics, and drugs. Various champaca plants in Indonesia have not been studied as one of Indonesia's biodiversity that can be used as excellent genetic resources (germplasm). The objective of the study was to determine the genetic diversity of champaca in Bali. The results revealed that 12 (twelve) champaca accession morphologically was characterized. All of accessions obtained from cultivation centers champaca in Bali. Based on the characteristic was observed by morphological characters i.e.: (a) Cempaka Putih Wilis (b) Cempaka Putih Pateh, (c) Cempaka Putih Patemon, (d) Cempaka Putih Sibang, (e) Cempaka Kuning Muda Petemon, (f) Cempaka Kuning Kecil Patemon, (g) Cempaka Kuning Besar Patemon, (h) (i) Cempaka Kuning Kecil Sibang, (j) Cempaka Kuning Tua Sibang, (k) Cempaka Kuning Muda Sibang, and (l) Cempaka Kuning Punah Sibang. Morphologically, champaca in Bali can be grouped into 4 clusters and therefore, and based on RAPD analysis champaca in Bali could be grouped into 5 clusters.
Keywords: champaca, identification, genetic, diversity, Bali
INTRODUCTION
Champaca plants have been planted in Indonesia, especially in Bali as a commercial commodity, thus it support economically for Bali communities. These plants have been utilized as ceremony purposes and worship for Hindu’s people. Others, the champaca flower were used for beauty salon, aromatic ingredients, and SPA. Taxonomically, champaca were planted in Bali are Magnolia alba and Magnolia champaca. The fragrant
aromatic of the flower is to be the specific characters of the plant.
As a tropical plant, champaca has a wide adaptation range in all tropical area from low land to highland. Usually, this family spread in warm subtropical of East Asia, Southeast Asia, and southeastern part of North America, West India, Central America (Kundu, 2009). In Bali, usually champaca was used as landscape element of yard and roadside, even in others part of Indonesia, champaca
ISSN ONLINE: 9 772303 337 008
timber was used as building material, such as for Balinese Hindu sacred building.
Beside of that uses, champaca flower was utilized for industrial raw materials, such as essential oils (Sanimah et al., 2008; Punjee et al., 2009; Krisdiana, 2010), parfumes, cosmetics, and drugs (Zumaidar, 2009; Armiyanti et al., 2010). The content of essential compounds that existed at plants champaca organs have also been used as an object of research. Research by Normansyah et al. (2013) showed that 80% ethanol extract of yellow champaca containing terpenoids and flavonoids compounds. Ahmad et al. (2011) analyzed using HPTLC (high performance thin layer chromatography) found that the quercetin compound (a flavonoid) in champaca leaf is higher than in the bark. Flowers of yellow champaca identified to have the ability of antioxidant and antimicrobial activity (Kumar et al., 2011), and flowers of white champaca is also identified to has antioxidant and antifungal activity (Bawa, 2011), while Ananthi and Chitra (2013) examined the antioxidants of champaca flower by in-vitro method.
Champaca plant that was planted in Indonesia has many various phenotypic, as exposed by vegetative and generative organs. Vary of champaca accession usually named by character of their organ, such as color of flower. Diversity of champaca plant will continue to increase in accordance with the trait of champaca plants that can be propagated by seed. Propagation by seed causes variations in champaca traits. According to Orwa et al. (2009) champaca crosspollination can be assisted by beetles.
Champaca plant varieties needs to be identified in order to determine their excellence properties that can be used as the basis of selection on further breeding programs. Generally, identification based on morphological traits (shape and structure) easy and fast, however identification by such morphological traits has many constraints, it can be affected by environment, different of plant age, and plant tissue (Khanuja et al., 2005). Plant identification that only base on morphological traits is difficult to apply on seedling stage.
Identification is very important to inventory and identify many champaca accessions that found and
cultivated in Bali and it is an effort to genetic germplasm conservation and plant development in the future. First step is morphological identification and genetic identification.
The aim of the study is to find out genetic variability of many champaca accessions and to assess the relationship between morphological and genetic traits of champaca accessions.
MATERIALS AND METHODS
This research was carried out at central of cultivation area in Bali, that are Sibang Gede and Sibang Kaja, Badung Regency and some areas that champaca was found. Morphological identification was done by inventory and observes important plant parts, such as leaf and flower. The plant parts were collected and measured their entire dimension.
After identification of morphological trait was done, genetic identification was carried out by using RAPD method. Young leaves were used as material of RAPD analysis. Centrifuge, digital balance, freezer, micro pipet, PCR machine, electrophoresis machine, UV transilluminator, digital camera, vortex,
thermometer, scissors, knife, ice box, and stationary.
Leaves were washed by tap water until clean and dried by tissue, and 0.1 g fresh sample were used. Sample was blended by mortar and added 800 µl CTAB buffer solution. CTAB buffer solution consist of CTAB 2%, 1,4M NaCl, 100 mM Tris HCl pH 8, 20 mM EDTA pH 8, 1% PVP-40 1, 1% mercaptoethnol, and kept under 65oC for 30 minutes. Mix solution incubated under 65˚C for 60 minutes. To homogenize the mixture was mixed for 10 minutes.
After incubated for 60 minutes mixtures were kept for 2 minutes then added for every 500 µl samples by 24 chloroform mixture : 1 isoamil alcohol (CIAA) and vortex for 5 minutes then centrifuged for 15 minutes at 12,000 rpm. Supernatan was taken and added cold isopropanol by 2/3 total (supernatant + sodium acetate), then mixed by shake and kept at -20˚C for minimum 1 hour. Afterward, it centrifuged at 12,000 rpm for 10 minutes. Supernatant was taken and DNA sediment washed by 500 µl ethanol 70% then centrifuged for 5 minutes at 12,000 rpm. Supernatant was taken and DNA sediment was
ISSN ONLINE: 9 772303 337 008
dried. After dry, DNA sediment was diluted again by 50 – 100 µl TE buffer + 1% RNAse in waterbath at 37˚C for 60 minutes. The incubation product was kept at refrigerator at 4oC.
DNA purification was then quantified by GeneQuant to detect DNA concentration. Reference was set by used psdH2O. Dilution factor (psdH2O) about 1998 µl and 2 µl DNA poured into cuvette, then put into the GeneQuant.
Dilution was carried out to detect the concentration of DNA that suitable for amplification process. To get final volume of dilution, DNA solution was added of psdH2O therefore the DNA concentration suitable for PCR
Dilution formula:
V1 M1 = V2 M2
M1= concentration of DNA sample
-
V1= initial volume of sample will be diluted
M2= 2.5 ng/µl
-
V2= final volume
Dilution volume = V2 - V1
DNA amplification was carried out by PCR to amplify DNA sequent
based on primer. Primers were utilized in this experiment as shown in Table 1.
PCR was carried out at 10 µl total volume on every PCR tube. Each PCR consist of 5 µl PCR mix Go Taq® Green (Promega), 0.25 µl 100 µM primer (Sigma-Proligo), 2.5 µl DNA sample (template) and 2.25 steril aquabidest.
Tabel 1. Concentration and purity DNA
|
No. |
Sample |
DNA concentration (ng/ µl) |
DNA purity |
|
1. |
Putih Wilis |
2250 |
1.731 |
|
2. |
Putih Pateh Putih |
1800 |
1.333 |
|
3. |
Patemon |
2050 |
1.206 |
|
4. |
Putih Sibang Kuning Muda |
3650 |
1.352 |
|
5. |
Patemon Kuning Kecil |
1850 |
1.542 |
|
6.. |
Patemon Kuning Besar |
4000 |
1.455 |
|
7. |
Patemon Kuning Kecil |
1800 |
1.565 |
|
8. |
Sibang Kuning Tua |
950 |
1.462 |
|
9. |
Sibang Kuning |
1100 |
1.692 |
|
10. |
Besar Sibang Kuning |
300 |
1.200 |
|
11. |
Muda Sibang Kuning Punah |
1400 |
1.867 |
|
12. |
Sibang |
1100 |
2.000 |
Tabel 2. Primers were used to DNA amplification of champaca
Primer Nucleotide base sequent OPA 5 5’ AGGGGTCTTG 3’
OPA 7 5’ GAAACGGGTG 3’
DNA amplification was done by PCR System BOECO equipment. First denaturation was done at 95oC for 1 minute, then followed by 45 cycles with temperature at 95˚C for 45
seconds, annealing at 37oC for 1 minute, and elongation at 72˚C for 1 minute and 30 seconds, then followed by final elongation at 72oC for 7 minutes.
DNA of PCR result then
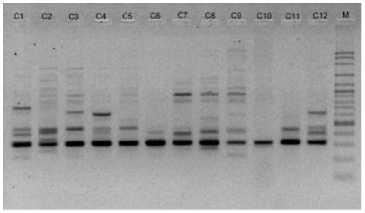
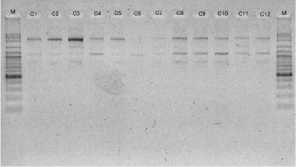
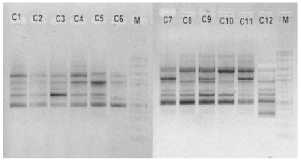
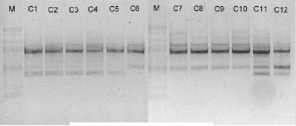
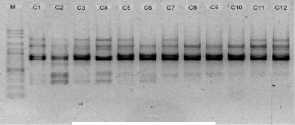
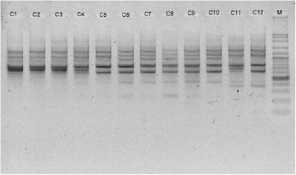
electrophoreses by 1,0 % (b/v) agarosa that was added ethidium bromide, in TBE buffer (consist of 0,45 M Tris-HCl pH 8, 0,45 M Boric acid, 20 mM EDTA) 100 volt in voltage for 45 minutes. Subsequently, it visualized by UV light, as shown in Figure 1.

OPA 5
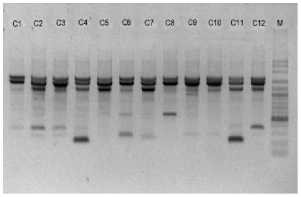
OPA 7
M C1 C2 C3 M C5 CS C7 C8 C9 ClO CU C12
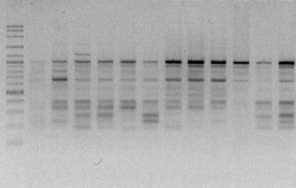
OPA 3
OPA 8
OPB 7
OPB 5
OPB 8
OPC 1
ISSN ONLINE: 9 772303 337 008
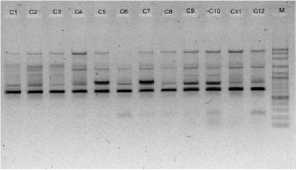
OPC 2
OPC 4
Figure 1. DNA amplification result of champaca by ten primers
Data was found by scoring of electrophoresis result to each sample at certain size, if presence of band was scored 1 and if absence scored 0. Binary data then analyzed by NTSYS-pc ver. 2.2.
RESULT AND DISCUSSION
Based on the results of field observations found that champaca plants cultivated as a cultivation plants are in Sibang Gede and Sibang Kaja, Badung Regency and Patemon, Buleleng Regency. Champaca spread all over Bali, sporadically from low land to highland.
Based on inventory result, champaca plant named based on flower color and their location. According to similarity and difference of champaca characters that were observed, it be discovered 12 champaca variances. Champaca plants grouped into 2 species that depend on color flower that
are white champaca and yellow champaca. Twelve variances were found as follows on Figure 2 and Table 3.
White champaca (Magnolia alba) has morphology trait as follows: up to 30 meters in high. On the branches of white champaca usually covered with grayish fine hairs. The leaves are oval and green. Petiole is quite long, reaching almost half the length of the leaves. The white champaca also has white flowers that have a distinctive fragrance (Orwa, 2009; Flora of China, 2008).
Morphological trait of yellow champaca plants (Magnolia champaca) is as follows (Orwa, 2009; Flora of China, 2008). Yellow champaca trees can reach a height of 15-25 m. Ends of twigs are hairy. The leaves are ovate lanceolate shape, with a pointed tip and base, 10-28 x 4.5 to 11 cm, thin as the skin.
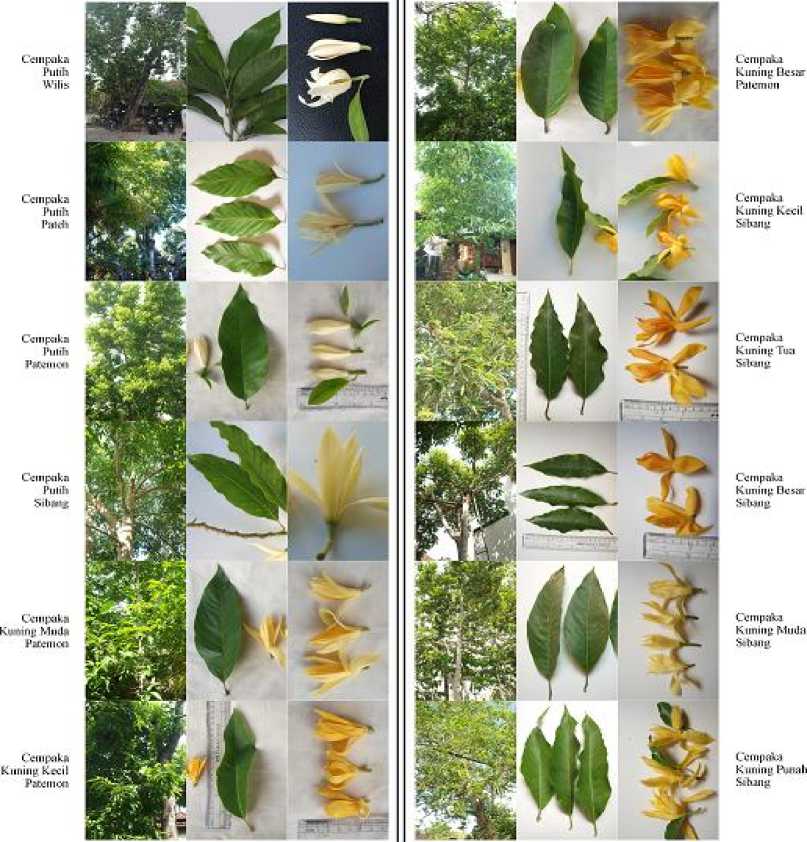
Figure 2. Twelve variances of champaca found in Bali
Table 3. Twelve variances of champaca found in Bali and their traits
|
No. Variance |
Name |
Specific Trait |
|
1. |
Cempaka Putih Wilis |
Flower color white; small sized |
|
2. |
Cempaka Putih Pateh |
Flower color white; small sized, huge habitus |
|
3. |
Cempaka Putih Patemon |
Flower color white; medium sized, leaf as Cempaka Putih Sibang |
|
4. |
Cempaka Putih Sibang |
Flower color white; large sized |
|
5. |
Cempaka Kuning Muda Patemon (Putih Cenana) |
Flower color yellowish white; smooth tepal |
|
6. |
Cempaka Kuning Kecil Patemon |
Flower color yellow; small sized |
|
7. |
Cempaka Kuning Besar Patemon |
Flower color yellow; large sized |
|
8. |
Cempaka Kuning Kecil Sibang |
Flower color yellow; small sized |
|
9. |
Cempaka Kuning Tua Sibang |
Flower color dark yellow; large sized |
|
10. |
Cempaka Kuning Besar Sibang |
Flower color yellow; large sized |
|
11. |
Cempaka Kuning Muda Sibang |
Flower color yellowish white; smooth tepal |
|
12. |
Cempaka Kuning Punah Sibang |
Flower color pale yellow; unsmooth tepal |
ISSN ONLINE: 9 772303 337 008
Scared stipule of the petiole length is more than half of the petiole.On the base of the pole-shaped flowers, ovaries and stamens clearly separated by a space. Ovary is more than 20, squeezed, flattened egg shape, hairy, each with some ovules. Fruit shape is spherical elongated, slightly bent, first green then pale gray, covered with acne. Ripe seeds are dark red hanging out on the beam that extends into a slender thread.
Morphologically, champaca can be grouped into 5 clusters. First cluster consist of 1 accession i.e. Cempaka Putih Wilis, Cluster-2 consist of 4 accession i.e. Cempaka Putih Pateh, Cempaka Putih Patemon, Cempaka Kuning Muda Sibang, and Cempaka Kuning Muda Patemon.
Cluster-3 consist of 1 accession
i.e Cempaka Putih Sibang. Cluster-4 consist of 4 accessions i.e. Cempaka Kuning Kecil Patemon, Cempaka Kuning Kecil Sibang, Cempaka Kuning Tua Sibang, and Cempaka Kuning Besar Sibang. Cluster 5 consist of 2 accessions i.e. Cempaka Kuning Besar Patemon and Cempaka Kuning Punah Sibang. The dendrogram of morphological trait as shown on Figure 3.
After identify genetically by RAPD method by using 10 primers, champaca found in Bali can be clustered into 4 clusters. Cluster-1 consist of 1 accession i.e. Cempaka Putih Wilis. Cluster-2 consist of 4 accessions i.e. Cempaka Putih Sibang, Cempaka Kuning Muda Patemon, Cempaka Kuning Muda Sibang, and Cempaka Kuning Punah Sibang.
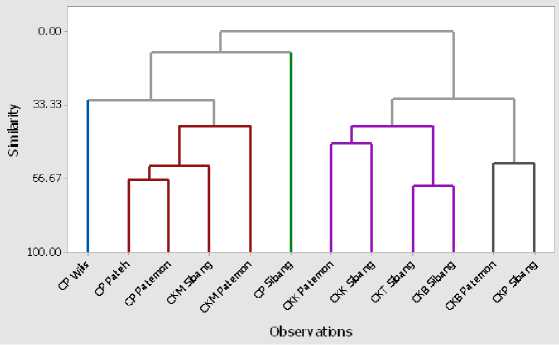
Figure 3. Dendrogram based on morphological trait of champaca found in Bali
ISSN ONLINE: 9 772303 337 008
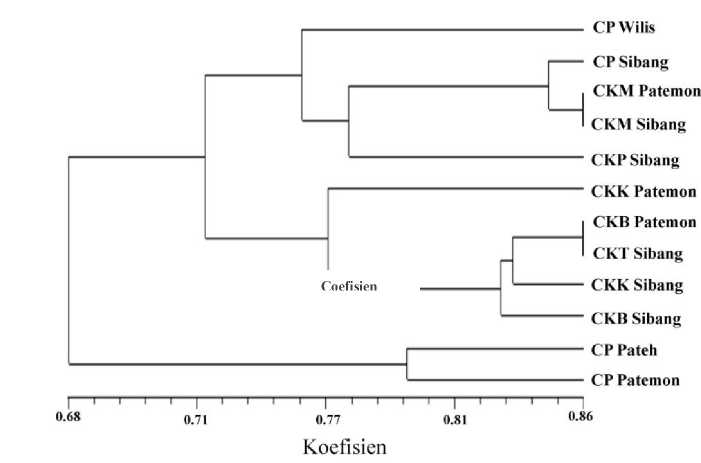
Figure 4. Dendrogram of genetic distance of 12 champaca found in Bali
Cluster-3 consist of 5 accessions i.e. Cempaka Kuning Kecil Patemon, Cempaka Kuning Besar Patemon, Cempaka Kuning Tua Sibang, Cempaka Kuning Kecil Sibang, and Cempaka Kuning Besar Sibang. Cluster-4 consist of 2
accessions i.e Cempaka Putih Pateh and Cempaka Putih Patemon. Dendrogram of genetic analysis by RAPD method shown in Figure 4. Morphologically, champaca in Bali can be grouped into 4 clusters and therefore, based on RAPD analysis champaca in Bali could be grouped into 5 clusters.
REFERENCES
Ahmad, H., A. Mishra, R. Gupta, dan
S. A. Saraf. Determination of
quercetin in Michelia champaca L. (Champa) leaves and stem bark by HPTLC. International Journal of Pharma and Bio Sciences, 2(4): 388 – 397.
Ananthi, T. and M. Chitra. 2013. In vitro evaluation of antioxidant activity of Michelia champaca (L.) flowers. American Journal of Advanced Drug Delivery, 1(5): 734 – 742.
Armiyanti, M. A. Kadir, S. Kadzimin, dan S. B. Panjaitan. 2010. Plant regeneration of Michelia champaca L., through somatic embryogenesis. African Journal of Biotechnology, 9(18), pp. 2640 – 2647.
Bawa, I G. A. G. Aktivitas antioksidan dan antijamur senyawa atsiri bunga cempaka putih (Michelia alba). Jurnal Kimia, 5(1): 43 – 50.
Flora of China. 2008. Michelia Linnaeus, Sp. Pl. 1:536.1753. Flora of China 7: 77-90
Khanuja, S. P. S., Shasany, A. K., Pawar, A., Lal, R. K., Darokar, M. P., Naqvi,A. A., Rajkumar, S., Sundaresan, V., Lal, N. and
Kumar, S. 2005. Essential oil constituents and RAPD markers to establish species relationship in Cymbopogon Spreng. (Poaceae). Biochemical
Systematics and Ecology, 33(2): 171 – 186.
Krisdiana, D. 2010. Isolasi, Karakterisasi, Identifikasi
Komponen, dan Uji Aktivitas Minyak Atsiri Bunga Cempaka Putih (Michelia alba) terhadap Escherichia coli dan
Staphylococcus aureus.
[http://karya-ilmiah.um.ac. id/index.php/ kimia/article/view/6168].
Diakses pada tanggal 18 Agustus 2013.
Kumar, R. V., S. Kumar, S.
Shashidhara, S. Anitha, dan M. Manjula. 2011. Antioxidant and antimicrobial activities of various extracts of Michelia champaca Linn flowers. World Applied Sciences Journal, 12 (4): 413-418.
Kundu, M. 2012. Michelia champaca Linn. Seed Leaflet, No. 158 Agustus 2012. Tropical Forest Research Institute. Forest & Landscape Denmark, University of Copenhagen.
Kundu, S. R. 2009. A synopsis on
distribution and endemism of Magnoliaceae s.l. in Indian
subcontinent. Thaiszia - J. Bot., 19: 47 – 60.
Mansyah, E., Baihaki, A.,
Setiamihardja, R., Darsa, J. S. dan Sobir. 2003. Analisis
variabilitas genetik manggis
(Garcinia mangostana L.) di
Jawa dan Sumatera Barat menggunakan teknik RAPD. Zuriat, 14 (1): 35 – 44.
Normansyah, A., Ariantari, N. P., Astuti, K. W. 2013. Profil kandungan kimia ekstrak etanol 80% kulit batang Michelia champaca L. dengan
kromatografi lapis tipis dan pereaksi pendeteksi. Jurnal Farmasi Udayana, 2(3): 153 – 156.
Orwa C, Mutua A , Kindt R , Jamnadass R, Simons A. 2009. Agroforestry Database: a tree reference and selection guide version 4.0 (http://www. worldagroforestry.org/af/treedb/ ) Diakses tanggal 18 Pebruari 2014.
Punjee, P. U. Dilokkunanant, U. Sukkatta, S. Vajrodaya, V. Haruethaitanasan, P.
Pitpiangchan, and P.
Rakthaworn. 2009. Scented extracts and essential oil extraction from Michelia alba D.C. Kasetsart J. (Nat. Sci.), 43: 197 – 203.
Sanimah, S., R. Suri, R. Nor Azizun, A. Hazniza, M. Radzali, I. Rusli, and M.D. Hassan. 2008. Volatile compounds of essential oil from different stages of Michelia alba (cempaka putih) flower development. J. Trop. Agric. and Fd. Sci., 36(1): 109 – 119.
Zumaidar. 2009. Kajian cempaka kuning (Michelia champaca L.) sebagai tumbuhan obat. J. Floratek, 4: 81 – 85.
38 • ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM
Discussion and feedback