CLONING OF FLOWERING GENES (WJFLC AND WJFT) IN WASABI (JAPANESE HORSERADISH) AND MONITORING OF FLOWERING RESPONSE WITH THEIR EXPRESSION
on
ARTICLE
CLONING OF FLOWERING GENES (WJFLC AND WJFT) IN WASABI (JAPANESE HORSERADISH) AND MONITORING OF FLOWERING RESPONSE WITH THEIR EXPRESSION
Masayuki Nozue1*, Hiroyoshi Kubo2 and Kiyoshi Yoshida3 1Division of Applied Biology, Faculty of Textile Science and Technology, Shinshu University, Tokida 3-15-1, Ueda 386-8567, Japan 2Department of Biology, Faculty of Science, Shinshu University, Asahi 3-1-1, Matsumoto 390-8621, Japan 3Marui Co. Ltd., Toyoshina 4932, Azumino 399-8205, Japan *Corresponding author: msnozue@shinshu-u.ac.jp
ABSTRACT
Wasabi (Wasabia japonica) is a commercially important crop in Japan. We isolated a FLC ortholog (WjFLC) and FT ortholog (WjFT) from wasabi. Predicted amino acid sequence encoded by WjFLC and WjFT showed 89% and 85% identities with FLC and FT of Arabidopsis, respectively. The expression of WjFLC was high in October and reduced in November when flower buds are formed in wasabi. On the other hand, expression of WjFT was not detected in October and was slightly detected in November. Thereafter, WjFT was highly expressed later in February. We examined the best condition for initiation of flower bud formation under various artificial environments by monitoring the flowering response of wasabi using WjFLC and WjFT.
Keywords: expression, flowering genes, flowering response, wasabi
INTRODUCTION
Wasabi (Wasabia japonica), a member of the Brassicaceae, is a commercially important crop in Japan. Wasabi is cultivated in restricted regions such a cool mountain streams or fields of highland because wasabi grows at relatively low temperature and low light. Growth conditions such light and temperature, have been examined from a horticultural point of view in wasabi (Tanaka et al., 2008; Tanaka et al., 2009). However, the physiological characters in flowering are poorly understood. The expression pattern of flowering-related genes could be used as a monitor in order to determine the best condition for flowering under artificial environment.
Flowering is controlled by various environmental factors, including low temperature and photoperiod (Jung and Müller, 2009; Amasino and Michaels, 2010). The molecular mechanism of the flowering has been well examined in the winter-annual plant Arabidopsis. Vernalization induces flowering by suppressing the expression of FLOWERING LOCUS C (FLC), which is a key repressor of
flowering (Michaels and Amasino, 1999; Sheldon et al., 2000). In Arabidopsis, CONSTANS (CO) is a key transcriptional regulator of the photoperiod pathway (Suárez-López et al., 2001). CO induces the expression of FLOWERING LOCUS T (FT), which is an integrator of floral-inductive signals from various genetic pathways (Helliwell et al., 2006). The expression of FT occurs in the leaf phloem, and FT protein moves to the meristem (Corbesier et al., 2007) and promotes floral transition through interaction with FLOWERING LOCUS D(FD) (Abe et al., 2005).
Under natural conditions, differentiation of flower buds in wasabi occurs between October and January and ceases in February. Inflorescence starts to grow in early spring and flowers open. After flowering, wasabi grows vegetatively until the following autumn. This perennial feature of flowering is distinct from that of annual and biennial Brassicaceae, which die after flowering. Although flowering is an interesting subject, there is little information on flowering genes in wasabi. In this study, we cloned FLC and FT orthologs from wasabi and examined their expression in the plants grown in the conditions both field and artificial environment.
MATERIALS AND METHODS
Rosette leaves of wasabi plantswere harvested from a field at Nakatou in Matsumoto, Japan on July 2, October 9, November 18, 2009 and February 13, 2010 and used for isolating RNA. The average temperature in early July, early October, middle of November and middle of February was 22°C, 14°C, 6°C and 0°C, respectively. Total RNA was isolated by the standard phenol-SDS methods.
Cloning of FLC ortholog (WjFLC) and FT ortholog (WjFT) from wasabi carried out usingthe procedures described in previous papers (Kubo et al., 2011; 2012). Semi-quantitative RT-PCR was performed to detect the expression of WjFLC and WjFT (Kubo et al., 2011; 2012).
For heterologous expression of WjFT in Arabidopsis, the cDNA fragment between the start codon and the stop codon was amplified by PCR. The PCR product was inserted into a modified version of binary vector pIG121-Hm. The plasmid was transferred to Agrobacterium tumefaciens C58C1 by electroporation method. Arabidopsis thaliana Columbia was grown as described previously (Kubo et al., 1999). Arabidopsis was transformed by vacuum infiltration. Transformed plants were selected with an agar medium containing kanamycin and carbenicillin. T1 seedlings were grown in soil and used to count leaf numbers.
RESULTS AND DISCUSSION
We isolated a FLC ortholog (WjFLC) from wasabi (DDBJ/GenBank/EMBL accession no. ADK92387). The coding region was 594 bp, which encoded a protein with 198 amino acid residues. The amino acid sequence predicted from the cDNA showed 89% identity with AtFLC (Fig. 1). MADS box domain signature was found between 3rd and 57th amino acids by PROSITE search. All the amino acid residues in the MADS box domain were conserved between AtFLC and its ortholog in wasabi.
HjFLC 1 MGRKKLElKElBHKssscvTmKRNGLlEKaKQLsvLcDMvALLvvsaSGKLreFSSG t**********"*.*********** + *************. ***.********** * **1k****.
AtPLC 1 MGRKKLEIKRIEKKSSRQVTFBKRRNGLIKKMIQLSVLCDaSVALLVVSASGKLYSPSSG
wj FLC 61 DnlvkildrVc-KqhvddlkaldlsskalnygShhellewr skive snvdnvsvdfi,aqγ, ⅛⅛* * ≠*≠*⅛≠* t∙⅛* _ *⅛⅛** ≠ * ⅛⅜,⅛⅛(**** *⅜⅛tτ⅛ +⅛*⅜ ***τ*⅛⅜ι* *_**
AtFLC 61 DNLVKILDRVGKQHaDDLKALDHQ S K-ALNlrG SHYELLE LVD S KLVGSNVKtTVS IDALVQL
HjFLC 151 Edhletslsltrahktelmlklvdslkekekmlkeenqvlasqmeknhhvgaeadnmeis * ⅛Λ⅛⅛**÷-**τp**⅛⅛⅛⅛±⅛⅛ r*rt⅛t⅛⅛Λ*∙***⅛**⅛rt*≠⅛⅛ι⅛⅛Λ**Λ⅛⅛ i* _ *
AtFLC 121 Eehletalsvtrakktelmlklvbnlkekekmlkbenqvlasqmennhhvgaeae-Mems
wjFLC 181 Frqisdinlpvtlpldn
AtPLC 180 Pagqisdnlpvtlpllk
Fig. 1. Comparison of amino acid sequences of WjFLC and AtFLC (Kubo et al., 2012). Identical and similar residues are indicated by asterisks and dots, respectively.
A FT ortholog (WjFT) was also identified in wasabi (DDBJ/GenBank/EMBL accession no. HQ667761). A cDNA encoding a protein with 175 amino acid residues was identified. Eighty– five percent of the amino acids was identical as between WjFT and AtFT.
⅛∣j∏, 1 Msisprdplwgrwtdvlepftrsislrvtyvcrwtngldlbpsqllnkprveigged
**t ∙at* » ∙t∙ ∙∙* »• t∙* t tt∙ ta aaaaaataata ***t**t∙ta*
MFT i Msinirdplivsrwgdvldpfnrsitlkvtygqrevtngldlrpsqvqnkprveigged
WjFT 61 LRNFYTLVHVDPDVPSPSNPHLREYLHWLVΓDIPλTTGTNFGNEIVSYESPRPTSGIHRI
HttttHnHt^Httt4Hl∣∣Ut<÷>t**HtOl>*ttH,til>,tt,H*tl
MFT 61 Lrnfytlvmvdpdvpspsnphlreylhwlvtdipawgttfgneivcyenpsptagihrv
WjFT 121 Vlvlfrqlgrqtvyepgwrqhfntrefaaiynlglpvaavffncqresgcggrrs * *a*∙ttt*t∙t aataa at*ataa atataaaaaat# ta∙*aaatt**a
AtFT 121 Vfilfrqlgrqtvyapgwrqnfntrefaeiynlglpvaavfyncqresgcggrrl
Fig. 2. Comparison of deduced amino acid sequences of WjFT and AtFT (Kubo et al., 2011). Identical and similar residues are indicated by asterisks and dots, respectively.
Flowering was promoted remarkably when 35S::WjFT was introduced into Arabidopsis (Fig. 3). In some transformed plants, inflorescence appeared when four rosette leaves formed (Fig. 3A). The wild type plants never formed inflorescence at the same stage. The average number of rosette leaves for the wild type was 12.4 under continuous light, but that of the T1 plants of the WjFT overexpressor (WjFTOX) was 5.4 (Fig. 3B). Strong expression of WjFT was observed in three representative WjFTOXlines (lines 25, 27 and 30) that showed the early-flowering phenotype (Fig. 3C). This indicates that WjFT is a promoter of flowering.
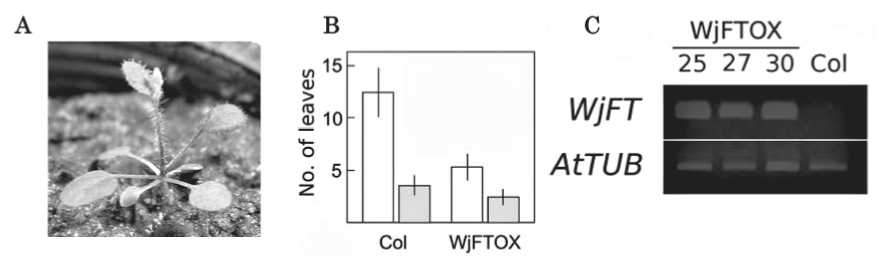
Fig. 3. Heterologous expression of WjFT in Arabidopsis (Kubo et al., 2011). A, This photograph shows a representative line that has two cotyledons, four rosette leaves, and two cauline leaves. B, Average numbers of rosette leaves (hollow box) and cauline leaves (solid box) were calculated from 32 wild type plants (Col) and 49 independent T1 plants of WjFToverexpressor (WjFTOX). Transformed plants selected with kanamycin (T1 seedlings) were grown in soil, and leaf numbers were counted. Bars indicate SD. C, The expression of WjFT in three WjFTOX lines (line 25, 27 and 30) and Col were examined by semi-quantitative RT-PCR. The expression of tubulin (AtTUB) was used as control.
The expression of WjFLC was examined in various parts of plant. Wasabi was harvested on July 2 to extract RNA from root, apical part of lateral bud, young leaf and mature leaf. RNA was also extracted from young leaf, rhizome and root of plants grown in a growth chamber, too. WjFLC was expressed all the tissues examined
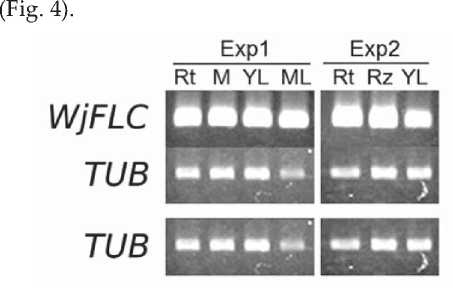
Fig. 4. Expression of WjFLC in various organs of wasabi (Kubo et al., 2012). Total RNA was extracted from root (Rt), apical region of lateral bud (M), young leaf (YL) and mature leaf (ML) of the plants grown in fields (Exp1), and root (Rt), rhizome (Rz) and young leaf (YL) of the plants grown in growth chamber (Exp2). The expression of WjFLC was examined by semi-quantitative RT-PCR. Tubulin (WjTUB) was used for control.
The expressions of WjFT and WjFLC were examined in the flowering process using wasabi grown in Nakatou area in Matsumoto, Japan. Wasabi grown in a field usually started to form flower buds in October when the average temperature was lower than 15°C. Therefore,
we expected that flower buds would be formed between October and November, because the average temperature around Nakatou area was 14°C in early October and 6°C in the middle of November. Rosette leaves were harvested from the plant grown in the field at Nakatou on October 9, 2009, November 18, 2009, and February 13, 2010 to examine the expression of WjFT and WjFLC (Fig. 5). The expression of WjFLC was high in October but reduced in November. In February, the expression of WjFLC could not be detected. Thus, the suppression of WjFLC expression seemed to be correlated with the induction of flowering, indicating WjFLC functions as a repressor as shown in AtFLC. On the other hand, WjFT was not detected in October, but increase in November. In February, the expression of WjFT was high (Fig. 5).
WjFT
WjFLC TUB
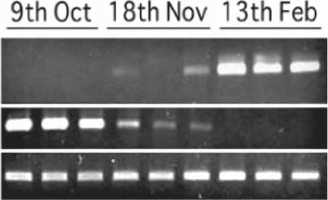
Fig. 5. Expression of WjFT and WjFLC in field grown wasabi plants (Kubo et al., 2011; 2012). Leaves were harvested on October 9 and November 18, 2009, and February 13, 2010. Different plants were used in different sampling dates. The expression of WjFT and WjFLC were examined by semi-quantitative RT-PCR. The expression of tubulin (WjTUB) was used as control. Experiments were triplicated from three different plants.
AtFLC has a central role in regulating the response to vernalization but several closely related homologs are identified in Arabidopsis (Ratcliffe et al., 2003). It is not known whether wasabi has paralogous genes other than the WjFLC. Because WjFLC was the most similar to AtFLC than the other FLC homologs of Arabidopsis, WjFLC may be a counterpart of AtFLC and play a central role in regulating the response to vernalization.
There is a possibility that wasabi has other WjFT paralogs. In a preliminary experiment, several putative FT homologs were identified in wasabi when PCR was performed using genome DNA as template. However, WjFT may play a central role in flowering because WjFT is the most similar to FT among the FT
homologs of Arabidopsis. Overexpression of WjFT remarkably promoted the flowering of Arabidopsis. Furthermore, the expression of WjFT increased in the season when wasabi forms flower buds. As the environmental condition for flowering has not been established in wasabi, WjFLC and WjFT could be useful in determining the best condition for flowering.
The expression of WjFLC was examined in 2-month–old and 6-month-old wasabi plants after seeding to determine the best condition for initiation of flower bud formation under artificial environment in the closed growing system. The suppression of WjFLC expression was not observed in 2-month-old plant grown at 10°C during 4 months (Fig. 6A), and no flower bud formation occurred. On the other hand, WjFLC
A
IM
2M
3M
4M
18LD 18SD IOLD IOSD
WjFLC TUB
WjFLC
TUB
WjFLC TUB
WjFLC
TUB
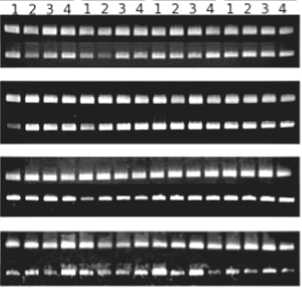
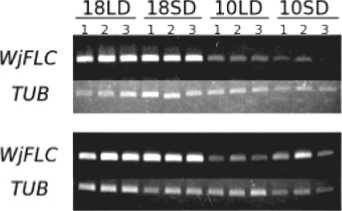
Fig. 6. Expression of WjFLC in wasabi plants grown under various artificial conditions in the closed growing system. Plants were grown for 2 months (A) and 6 months (B) at 18°C under 12hL:12hD after seeding, and then transferred to 18°C under long day (16hL:8hD) (18LD), 18°C under short day (8hL:16hD) (18SD), 10°C under long day (16hL:8hD) (10LD) and 10°C under short day (8hL:16hD) (10SD) for 1 to 4 months, respectively. Total RNA was extracted from leaves of four (A) or three different plants (B). The expression of WjFLC was examined by semi-quantitative RT-PCR. The expression of tubulin (WjTUB) was used as control.
Table 1. Number of Flower buds formed in wasabi under various artificial environmental conditions during 3 months.
Artificial environmental condition
18LD 18SD IOLD IOSD
|
Plant’ |
1 |
2 |
3 |
1 |
2 |
3 |
1 |
2 |
3 |
1 |
2 |
3 |
|
Number of flower bud |
2 |
0 |
0 |
0 |
0 |
0 |
7 |
9 |
U |
0 |
0 |
1 |
’Plants were grown for 6 months at 180C under 12hL:12hD after seeding, and then three plants in each experiment were transferred to at 18oC under long day (16hL:8hD) (18LD), 18oC under short day (8hL:16hD) (18SD), 10oC under long day (16hL:8hD) (IOLD) and IO0C under short day (8hL:16hD) (IOSD) for 3 months, respectively.
expression in 6-month-old plant was markedly reduced at 10°C (Fig. 6B), and flower buds were formed at 10°C under long day (16hL:8hD) rather than short day (8hL:16hD) (Table 1). These results indicated that wasabi plant must reach a certain developmental stage to sense low temperature for initiation of flower bud formation. Exposure to low temperature more than one month is essential to suppress the expression of WjFLC in the flowering response in wasabi. The long day condition is also important for the expression of WjFT that is the strong promoter of flowering.
ACKNOWLEDGEMENTS
This research was supported by the Program for Fostering Regional Innovation in Nagano, granted by MEXT, Japan. This work was also supported by a Grants-Aid for Scientific Research (24580367) from MEXT, Japan. We are grateful to Shinshu University Research Center for Advanced Plant Factory (SU-PLAF), for supporting the present study.
REFERENCES
Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K and Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052-1056.
Amasino RM and Michaels SD. 2010. The timing of flowering. Plant Physiol. 154: 516-520.
Corbesier LC, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C and Coupland, G. 2007. FT protein movement contributes to longdistance signaling in floral induction of Arabidopsis. Science 316: 1030-1033.
Helliwell CA, Wood CC, Robertson M, Peacock WJ and Dennis ES. 2006. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high–molecular-weight protein complex. Plant J. 46: 183-192.
Jung C and Müller AE. 2009. Flowering time control and applications in plant breeding. Trends Plant Sci. 14: 563-573.
Kubo H, Peeters AJM, Aarts MGM, Pereira A and Koornneef M. 1999. ANTHOCYANINLESS 2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell 11: 1217-1226.
Kubo H, Yoshida K and Nozue M. 2011. Cloning of a FLOWERING LOCUS Tortholog in Wasabia japonica (Matsum). Biosci. Biotechnol. Biochem. 75: 1823-1825.
Kubo H, Yoshida K and Nozue M. 2012. Cloning of FLOWERING LOCUS Cortholog in Wasabia japonica (Matsum.). J. Plant Biochem. Biotechnol. 21: 117-120.
Michaels SD and Amasino RM. 1999. FLOWERING LOCUS C encodes a novel MADS Domain protein that acts as a repressor of flowering. Plant Cell 11: 949956.
Ratcliffe OJ, Kumimoto RW, Wong BJ and Riechmann JL. 2003. Analysis of the Arabidopsis MADS AFFECTING FLOWERING gene family: MAF2 prevents vernalization by short periods of cold. Plant Cell 15: 1159-1169.
Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ and Dennis ES. 2000. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC). Proc. Nat. Sci. USA 97: 3753-3758.
Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F and Coupland G. 2001. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410: 1116-1120.
Tanaka I, Funahashi Y and Shimazu T. 2008. Study on cultivation of Japanese horseradish (Wasabia japonica Matsum.) using artificial light. Eco-Engineering 20: 119-124.
Tanaka I, Ito Y, Shinozuka M, Shimazu T. 2009. Indoor cultivation of Japanese horseradish (Wasabia japonica Matsum.) using
artificial light. Effects of air and solution temperatures on plant growth. J. Sci. High Tech. Agri. 21: 175-178.
udayana university biosciences and biotechnology forum • 71
Discussion and feedback