THE POSSIBLE ROLE OF CELL WALL DEGRADATION ENZYMES TO BACTERIOPHAGES INFECTION AND PRODUCTION (Review)
on
J. Agric. Sci. and Biotechnol.
ISSN: 23020-113
Vol. 1, No. 1, Juli 2012
THE POSSIBLE ROLE OF CELL WALL DEGRADATION ENZYMES TO BACTERIOPHAGES INFECTION AND PRODUCTION
(Review)
I Putu Sudiarta
Faculty of Agriculture, Udayana University Correspounding addres at: Jl. PB. Sudirman Denpasar 80362 Bali Email address: putu.ueda@yahoo.com
Abstrak
Penggunaan pestisida kimia sintetis untuk mengendalikan hama dan penyakit tumbuhan memiliki banyak pengaruh negatif, seperti ketahanan serangga hama dan penyakit terhadap pestisida, resurgensi, ledakan hama sekunder, punahnyah musuh alami dan residu pada bahan makanan dan lingkungan. Selama 60 tahun-an terakhir tingkat keracunan dibidang pertanian dan lingkungan semakin meningkat secara dramatis. Oleh karena itu salah satu pendekatan untuk mengurangi penggunaan pestisida kimia adalah menggunakan musuh alami. Untuk mengendalikan penyakit yang disebabkan oleh bakteri salah satu pendekatan mutahir menggunakan musuh alami berupa bakteriopag. Bakteriopag menyerang bakteri dengan kisaran inang yang spesifik. Salah satu penelitian yang menarik adalah tentang mekanisme bakteriopag menyerang inangnya. Untuk menghancurkan dinding sel bakteri, bakteriopag menghasilkan banyak ensim pendegradasi dinding sel. Pada kajian ini didiskusikan beberapa ensim pendegradasi dinding sel dan kemungkinan perannannya dalam mekanisme infeksi bakteriopag.
Key word: ensim pendegradasi dinding sel, pengendalian hayati, bakteriopag
The number of population and the food consumption is still increasing however the productive land of agriculture is decreasing. In solving this problem, in 1992 UNCED (United Nations Conference on Environmental and Development) recommended a concept of sustainable development (Djuniadi, 2003). Its recommendation is the sustainable development must has the attention to social, economic, and ecological aspect in the local, regional, and global level. On the other hand, the food production during the past decades 1940s has been conducted
to use the chemical intensively, such as chemical pesticides and fertilizers (Erickson 2009). The utilization of chemical pesticide releasing the negative effect, such as the insecticide resistance, resurgence, outbreak of secondary pests and diseases, disappearance of parasitoid and predator, residual effect of food and environmental. Over the past 60 years both the number of agricultural toxicants in the environment and rates of toxin-related diseases have increased dramatically. These ‘toxicants’ are widely distributed in nature and many lack an established NOAEL (no observed adverse effect level) or consensus about long term health effects (Oates and Cohen, 2009).
The one possible way to reduce the utilization of chemical pesticides in agriculture area is by the utilization of natural enemies. Naturally in ecosystem the pests and diseases have enemies, competitors and/or antagonists, such as parasitoids, predators, pathogens, viruses (bacteriophages) (Lacey et al., 2001; Loessner 2005; Susila et al, 2005; Supartha et al, 2005; Sumiartha et al, 2006;). In organic farming system to utilize of them is possible to make the ecosystem balance and sustainable.
One advance way to control diseases causing by bacteria is utilize the bacteriophages. The some study about the mechanism of infection of bacteriophages has been reported. Bacteriophage T7 was reported infect the Escherichia coli cells, and the role of gp16 cell wall lytic enzyme is beneficial during that infection (Moak and Molineux, 2000). Loessner (2005) reviewed about the application of bacteriophage endolysins (cell wall lytic enzymes). Endolysins in food and in biotechnology has been used, with the specific action. Therefore they offer a unique possibility for the biological control of unwanted bacteria without having any effect on other organisms, such as the natural flora. In medical
application the purified preparations of cell wall lytic enzymes could also be used astherapeutic agents, either alone or in combination with classical antibiotics, particularly in external applications (Loeffler et al, 2001; Loessner, 2005). However in my knowledge in agriculture area to control bacteria the information and application of bacteriophage cell wall lytic enzymes are limited. For more clear, in this review the possible role of cell wall lytic enzymes in the mechanism of infection of bacteriophahe will be summarized and discussed.
Cell wall lytic enzymes were reported play many important roles in the life cycle of bacteria, as well as vegetative growth, sporulation and germination. The roles of individual enzymes in each process have been studied by constructing mutant. The one of the best study about cell wall lytic enzymes has been conducted in Bacillus subtilis. B. subtilis is the best characterized member of the Gram-positive bacteria, has been studied for more than 50 years as a model of microorganism, in its biochemistry, physiology and genetics. Its genome of 4,215,810 base pairs comprises about 4,100 protein-coding genes, with the origin of replication coinciding with the base numbering start point, and terminus about 2,017 kilo bases. The genome contains at least ten prophages or remnants of prophages. B. subtilis is an aerobic, non-pathogenic bacterium, endospore-forming, rod-shaped bacterium, commonly found in soil, water, and in association with plants. (Kunst et al., 1997).
The involvement of cell wall degradation enzymes includes peptidoglycan maturation, cell separation, motility and competence, cell expansion, cell wall
turnover, protein secretion, differentiation, mother-cell lysis and pathogenicity (Fig. 1) (Foster 1992; Blackman et al., 1998; Smith et al., 2000).
-
B. subtilis has more than 30 cell wall lytic enzymes. The cell wall lytic enzymes in B. subtilis are classified according to their hydrolytic bond specificity as muramidases, lytic transglycosylases, glucosaminidases, amidases, and endopeptidases (Fig. 2) (Shida and Sekiguchi 2005, Sudiarta et al., 2010a, Sudiarta et al., 2010b). In applied science, cell wall lytic enzymes, as well as muramidase (lysozyme) have been utilized in wide area (biochemistry, agriculture, textile as well as food industry). In addition some of cell wall lytic enzymes were utilized in medical field, such as for controlling Staphylococcus aureus. The advent of S. aureus strains that are resistant to virtually all antibiotics has increased the need for new antistaphylococcal agents. An example of such a potential therapeutic is lysostaphin, an enzyme that specifically cleaves the S. aureus peptidoglycan, thereby lysing the bacteria (Kumar, 2008; Francius et al., 2008).
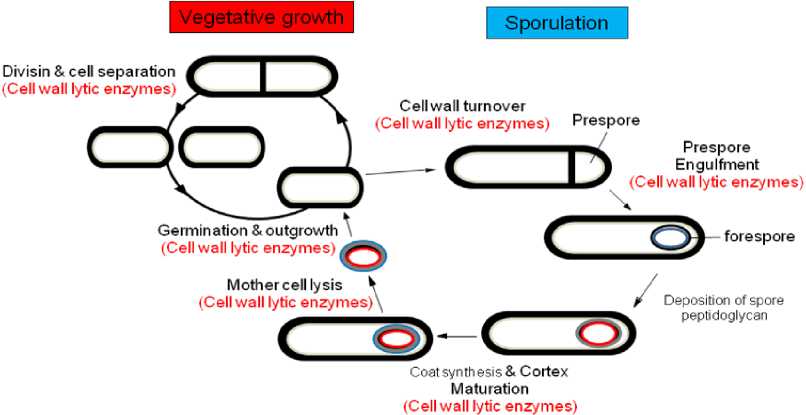
Figure 1. Life cycle of Bacillus subtilis. Important roles of cell wall lytic enzymes in life cycle of B. subtilis.
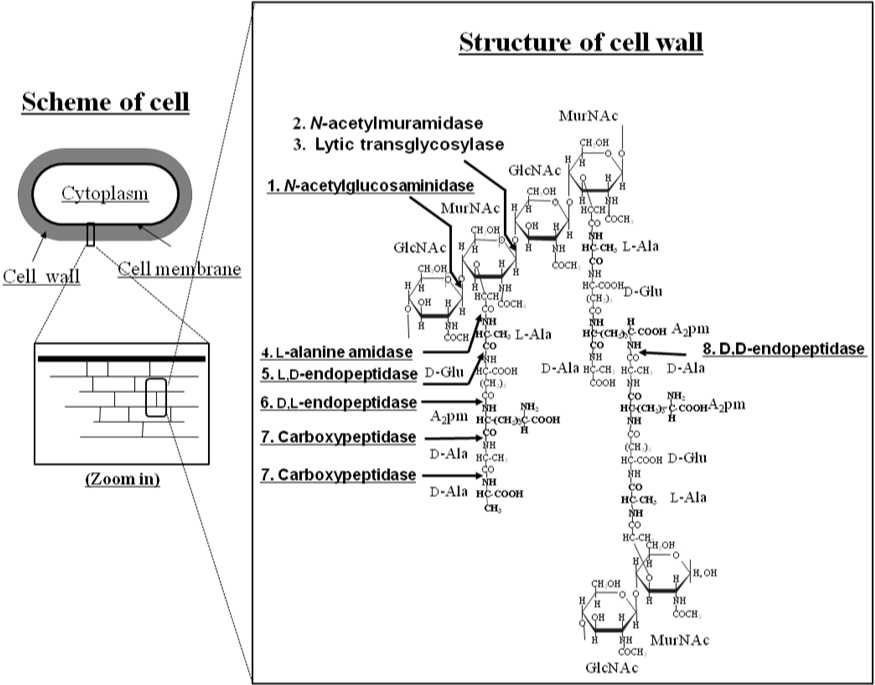
Figure 2. Structure of typical B. subtilis peptidoglycan of vegetative cells. The arrows indicate hydrolytic bonds attacked by cell wall hydrolases.
-
2.2 Propose the role of cell wall lytic enzymes in bacteriophages infection
On the other hand, the cell wall lytic enzymes are also reported to play important role in phage infection (Fig. 3). Cell wall lytic enzymes (endolysins) are phage-encoded enzyme that degraded the peptidoglycan of host at the terminal stage of the phage production (Loessner, 2005). The gp 13 of bacteriophage Ф29 has cell wall lytic activity and has very importance role for bacterial virus entry (Cohen et al., 2009).
The cycle of bacteriophages to infect the microorganisms seems to be simple; adsorption, insertion of nucleic acids, production of nucleic acids and proteins of bacteriophages, and host cell lysis, sequentially. However, the infection of suitable target microorganisms is very specific. Cell wall hydrolases encoded in bacteriophage genomes are involved in host cell lysis (final infection cycle) and in
adsorption (facilitation of infection) (first infection cycle) Fig. 3 (Piuri and Hatfull, 2006).
Recently, we reported the possible role of cell wall lytic enzymes (CwlP) related with phage infection in B. subtilis (Sudiarta et al., 2010b). The target protein, CwlP, is a phage-related protein whose gene is located in the SP-beta prophage (Regamey and Karamata, 1998). CwlP is the largest protein, comprising 2,285 a.a. (252 kDa), in the prophage region. Since CwlP has a phage-related minor tail domain, it is possible that the protein acts as a tail protein. Recently, Piuri and Hatfull (2006) described that gp17 of tape measure protein (Tmp) is the mycobacteriophage TM4 tail protein (1,229 a.a.). The protein contains a cell wall lytic enzyme, and that hydrolysis by the hydrolase facilitates efficient infection of stationary phase cells. Interestingly, Kenny et al. (2004) showed that Orf50 of bacteriophage Tuc2009 (906 a.a.) encodes tail-associated cell wall-degrading activity and is involved in infection through cell wall hydrolysis. To deliver the DNA and to break the cell wall of host completely in the end of life cycle, the bacteriophages were reported produced the cell wall lytict enzymes (Fig. 3).
Step 2. Prophage
Step3. Phage production
(Partial cell wall hydrolysis)
(Cell wall degradation by hydrolase)
Step 1. Insertion of phage DNA into cells host
Phage DNA
(Phage)
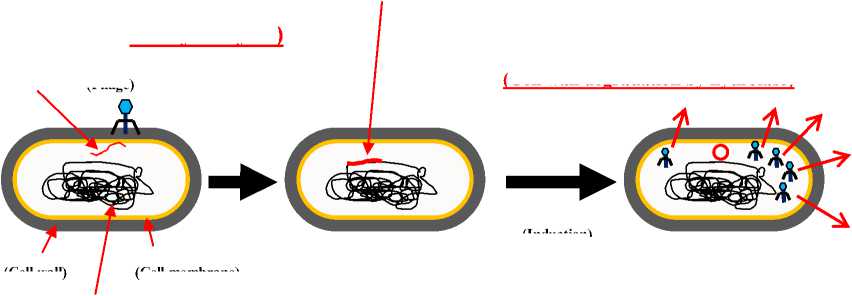
(Induction)
(Cell wall)
(Cell membrane)
(Chromosome)
Figure 3. Life cycle of lysogenic phage. Cell wall lytic enzymes play important
roles in life cycle of lysogenic phage, at least in the periods of phage DNA insertion into the host cell (step 1) and host cell lysis after phage production (Step 2).
To support the organic farming system and sustainable agriculture the biological control for pests and diseases is essential approach. The biological control by utilization of natural enemies is one of importance step to reduce the utilization of chemical. The advance approach to control diseases causing by bacteria is by bacteriophages. The high technology and depth of knowledge is needed for this way, however is to be simple if understand the specific step of infection mechanism of bacteriophage, as well as the role of cell wall lytic enzymes. That information will be necessary for basic and applied study, particularly to utilization of bacteriophages in agriculture area.
Acknowledgement
We express our thanks to Prof. Dr. J Sekiguchi, Dr. T. Fukushima, and Dr. T. Kodama for kindly supporting to discuses and all helps.
References
Blackman S.A., Smith T.J. and Foster S.J. 1998. The role of autolysin during vegetative growth of Bacillus subtilis 168. Microbiology 144: 73-82.
Cohen D.N., Sham Y.Y., Haugstad G.D., Xiang Ye, Rossmann M.G., Anderson D.L., and Popham D.L. 2009. Shared catalysis in virus entry and bacterial cell wall depolymerization. J. Mol. Biol. 387: 607-618.
Djuniadi D. 2003. Role of Industry on Integrated pest management on sustainable agriculture. Indonesian Association of Entomology Congress and Entomology Symposium VI 2003. Cipayung. 17-26. (In Indonesian Language).
Erickson, B. E. 2009. Next-generation risk assessment: EPA’s plan to adopt in vitro methods for toxicity testing gets mixed reviews from stakeholders. Chemical & Engineering News, 87: 30-33.
Foster S.J. 1992. Analysis of autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 174: 468-470.
Francius G., Domenech O., Mingeot-Leclercq M.P., and Dufrêne Y.F. 2008. Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J. Bacteriol. 190(24): 7904–7909.
Kenny J.G., McGrath S., Fitzgerald G.F., and van Sinderen D. 2004. Bacteriophage tuc2009 encodes a tail-associated cell wall-degrading activity. J. Bacteriol. 186: 3480-3491.
Kumar J.K. 2008. Lysostaphin: an antistaphylococcal agent Appl. Microbiol. Biotechnol. 80: 555-561.
Kunst F., Ogasawara N., Moszer I., Albertini A.M., Alloni G., Azevedo V., Bertero M.G., Bessieres P., Bolotin A., Borchert S., Borriss R., Boursier L., Brans A., Braun M., Brignell S.C., Bron S., Brouillet S., Bruschi C.V., Caldwell B., Capuano V., Carter N.M., Choi S.-K., Codani J.-J., Connerton I.F., Cummings N.J., Daniel R.A., Denizot F., Devine K.M., Duesterhoeft A., Ehrlich S.D., Emmerson P.T., Entian K.-D., Errington J., Fabret C., Ferrari E., Foulger D., Fritz C., Fujita M., Fujita Y., Fuma S., Galizzi A., Galleron N., Ghim S.-Y., Glaser P., Goffeau A., Golightly E.J., Grandi G., Guiseppi G., Guy B.J., Haga K., Haiech J., Harwood C.R., Henaut A., Hilbert H., Holsappel S., Hosono S., Hullo M.-F., Itaya M., Jones L.-M., Joris B., Karamata D., Kasahara Y., Klaerr-Blanchard M., Klein C., Kobayashi Y., Koetter P., Koningstein G., Krogh S., Kumano M., Kurita K., Lapidus A., Lardinois S., Lauber J., Lazarevic V., Lee S.-M., Levine A., Liu H., Masuda S., Mauel C., Medigue C., Medina N., Mellado R.P., Mizuno M., Moestl D., Nakai S., Noback M., Noone D., O'Reilly M., Ogawa K., Ogiwara A., Oudega B., Park S.-H., Parro V., Pohl T.M., Portetelle D., Porwollik S., Prescott A.M., Presecan E., Pujic P., Purnelle B., Rapoport G., Rey M., Reynolds S., Rieger M., Rivolta C., Rocha E., Roche B., Rose M., Sadaie Y., Sato T., Scanlan E., Schleich S., Schroeter R., Scoffone F., Sekiguchi J., Sekowska A., Seror S.J., Serror P., Shin B.-S., Soldo B., Sorokin A., Tacconi E., Takagi T., Takahashi H., Takemaru K., Takeuchi M., Tamakoshi A., Tanaka T., Terpstra P., Tognoni A., Tosato V., Uchiyama S., Vandenbol M., Vannier F., Vassarotti A., Viari A., Wambutt R., Wedler E., Wedler H., Weitzenegger T., Winters P., Wipat A., Yamamoto H., Yamane K., Yasumoto K., Yata K., Yoshida K., Yoshikawa H.-F., Zumstein E., Yoshikawa H., Danchin A. 1997.The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390, 249-256.
Lacey l.A., Frutos R., Kaya H.K., and Vai P. 2001. Insect pathogens as biological control agents: do they have a future? Biological Control 21, 230–248.
Loeffler J.M., Nelson D, Fischetti V.A. 2001. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294: 2170-2172.
Loessner M.J. 2005. Bacteriophage endolysins-current state of research and application. Current opinion in microbiology 8, 480-487.
Moak M. and Molineux I.J. 2000. Role of the Gp16 lytic transglycosylase motif inbacteriophage T7 virions at the initiation of infection. Molecular Microbiology 37(2): 345-355.
Oates L. and Cohen M. 2009. Human consumption of agricultural toxicants from organic and conventional food. Journal of Organic Systems 4 (1): 48-57.
Piuri M., and Hatfull G.F. 2006. A peptidoglycan hydrolase motif within the mycobacteriophage TM4 tape measure protein promotes efficient infection of stationary phase cells. Mol. Microbiol. 62: 1569-1585.
Regamey A., and Karamata D. 1998. The N-acetylmuramoyl-L-alanine amidase encoded by the Bacillus subtilis 168 prophage SPp. Microbiology 144: 885-893.
Shida T., Sekiguchi J. 2005. Cell wall degradation and modification hydrolases in Bacillus subtilis. Research signpost, survival and death in bacteria 117-142, ISBN: 81-7736-236-4.
Smith T.J., Blackman S.A. and Foster S.J. 2000. Autolysin of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146: 249-262.
Sudiarta I P., Fukushima T., and Sekiguchi J. 2010a. Bacillus subtilis CwlQ (previous YjbJ) is a bifunctional enzyme exhibiting muramidase and soluble-lytic transglycosylase activities. Biochemical and Biophysical Research Communications 398: 606–612.
Sudiarta I P., Fukushima T., and Sekiguchi J. 2010b. Bacillus subtilis CwlP of the SP-beta prophage has two novel peptidoglycan hydrolase domains, muramidase and cross-linkage digesting d,d-Endopeptidase. The Journal of Biological Chemistry. 285, ( 53): 41232–41243.
Susila, W., Sumiartha K., Okajima S., and Sudiarta P. 2005. Biological aspects studies and mass production method of the ectoparasitoid Hemiptarsenus varicornis (Girault) (Hymenoptera: Eulopidae) on Leaf Miner Play, Liriomyza sativae Blanchard (Diptera: Agromizidae). Paper Presented at the ISSAAS International Congress 2005, Hanoi Vietnam.
Sumiartha K., Susila W. and Sudiarta P. 2006. Biological study of egg rice yello stemborrer parasitoid (Tetrastichus schoenobii) (Hymenoptera: eulopidae) in the laboratory. Journal of ISSAAS 12(2): 74-75.
Supartha, I W., Bagus I G.N., and Sudiarta I P. 2005. Biodiversity of the population of Liriomyza spp. (Diptera: Agromyzidae) and Parasitoids in vegetables crop in high land area. Agritrop 24 (2): 43-51.
http://ojs.unud.ac.id/index.php/JASB
38
Discussion and feedback