Bio efficacy of frog skin bacteria as biological control agents against chili anthracnose disease
on
JURNAL BIOLOGI UDAYANA
P-ISSN: 1410-5292 E-ISSN: 2599-2856
Volume 27 | Nomor 1 | Juni 2023
DOI: https://doi.org/10.24843/JBIOUNUD.2023.v27.i01.p11
Bio efficacy of frog skin bacteria as biological control agents against chili anthracnose disease
Bioefikasi bakteri dari kulit katak sebagai agensia biokontrol terhadap penyakit antraknosa pada cabai
Lela Susilawati1,*, P. Afrizka Sari1, Maulana Septiani1, E.S. Purnomo1,2
-
1) Department of Biology, Faculty of Science and Technology, UIN Sunan Kalijaga, Yogyakarta
Jl. Marsda Adisutjipto No.1, Yogyakarta-Indonesia, 55281
-
2) Lab. Microbiology, Integrated Laboratory, UIN Sunan Kalijaga, Yogyakarta
Jl. Marsda Adisutjipto No.1, Yogyakarta-Indonesia, 55281
*Email: lela.susilawati@uin-suka.ac.id
Diterima
20 Maret 2023
ABSTRACT
Disetujui 16 Juni 2023
Amphibian skin e.g., frog carry bacterial symbionts on their skin that protect the frog from invasion of pathogen infection. This study aimed to evaluate antifungal activity of five bacterial skin frog (namely KSMD3; KSMD9; KSMD10; KSMV12; KSMV15) of Indonesian origin, Fejervarya limnocharis, against phytopathogen (Colletotrichum capsici) TCKr2. Primary screening for their antifungal activity was performed using dual culture method on nutrient agar contained 2% (w/v) of dextrose. The alteration of hyphal morphology on media and the detached chili fruit bioassay were observed. Isolate of KSMD3 was selected based on its significant performance in inhibiting the growth of the chili anthracnose pathogen, C. capsici. In addition, the KSMD3 showed low severity of disease incidence on detached chili fruit. Based on the analysis of 16S rDNA, the isolate of KSMD3 was identified as member of genera of Pseudomonas.
Kata kunci: antifungal activity, Colletotrichum sp. bioassay, Indonesian frog, Pseudomonas sp.
INTISARI
Kulit amfibi seperti katak membawa simbion bakteri pada kulitnya yang melindungi katak dari serangan infeksi patogen. Penelitian ini bertujuan untuk mengevaluasi aktivitas antifungi dari lima bakteri kulit katak yaitu KSMD3; KSMD9; KSMD10; KSMV12; KSMV15 yang disiolasi dari spesies katak asal Indonesia, Fejervarya limnocharis terhadap fitopatogen (Colletotrichum capsici) TCKr2. Skrining utama aktivitas antifungi dilakukan menggunakan metode dual culture pada media agar yang mengandung 2% (b/v) dekstrosa. Adanya perubahan morfologi hifa dan bioassay buah cabai diamati. Isolat KSMD3 terpilih berdasarkan aktivits antifungi dalam menghambat pertumbuhan C. capsici. Selain itu, KSMD3 menunjukkan tingkat keparahan penyakit yang rendah pada buah cabai. Berdasarkan analisis 16S rDNA, isolat KSMD3 diidentifikasi sebagai anggota genus Pseudomonas.
Kata kunci: aktivitas antifungi, Colletotrichum sp., bioassay, katak Indonesia, Pseudomonas sp.
INTRODUCTION
Amphibian skin is frequently exposed to the environment including physical factors, microorganisms and predators (Xu & Lai, 2015). The amphibian skin is relatively thin and permeable (Varga et al., 2019), hence, their skin is vulnerable to pollutants (McCoy & Peralta, 2018) and pathogen (Madison
et al., 2017). Therefore, the essential physiological processes occurred in their skin play an important role for maintaining their health (Varga et al., 2019).
Amphibian skin can secret some remarkable substances such as antioxidants (C. Liu et al., 2010), and antimicrobial peptides (AMPs) (Conlon, 2011). Those substances not only has the essential role for gas exchange but also host defense against some invasive organisms (Jared et al., 2018). Mostly, those discovered peptides from frog skin demonstrated high antimicrobial and antifungal activities (Abbassi et al., 2008) that effectively against Gram-positive and Gram-negative bacteria. In addition to several studies indicated that these peptides from frog skin considerably have attracted to be developed as a promising therapeutic alternative in the future. For example, frog’s peptide brevinine-1, brevinine-2, esculantin-2, and temporin from Hylarana erythraea (the green frog) (Al-Ghaferi et al., 2010) showed effectively inhibit the growth of Escherichia coli, Staphylococcus aureus, Candida albicans, and Acetinobacter baumannii. Recently D’Auria et al. (2022) reported that Temporin G (TG), originally isolated from Rana temporaria (Europian red frog), demonstrated inhibitory activity against fungi C. albicans and Cryptococcus neoformans. Moreover, TG showed high activity that inhibited the virulence factor of C. albicans.
These antimicrobial compounds are secreted by microbial symbiont on amphibian skin (Mangoni et al., 2001). These communities of microbiota occupies the mucous layer forming a microbiological barrier for skin protection (Smith et al., 2018; Varga et al., 2019). High diversity of microbes can grow and reside on amphibian skin surface because the skin provides a suitable habitats for them by producing a glycoprotein-containing mucus (Austin, 2000).
Previous studies have been reported of the recovery of frog-skin bacterial symbiont from several area and species. Belden et al. (2015) successfully isolated diverse species of bacteria from the skin of frogs, Craugastor fitzingeri (Fitzinger’s robber frog), Dendropsophus ebraccatus (pantless treefrog), and Agalychnis callidryas (red-eyed leaf frog) in Panama. From Japan, Susilawati et al., (2021) obtained a total 102 bacterial isolates from three native species of wild frog Hyla japonica, Pelophylax porosus porosus and Buergeria buergeri. Yet, to our knowledge, no study has been reported in Indonesian frog. Susilawati & Sari (2014) obtained 20 culturable bacteria isolates from local species of frog Fejervarya limnocharis with no further study on the identification and their potentiality against invasive pathogen.
In fact, Indonesia is one of the megadiverse countries in the world comprises about 17,000 islands with high diverse of endemic species of floras and faunas (Iskandar & Erdelen, 2006). This provides an opportunity to preserve species of frog in Indonesia by applying its microbial community for controlling other microbial pathogen such as bacteria, fungi and virus in agriculture, wildlife, and human. Here, we investigate the bacterial community on endemic Indonesian frog and evaluating their antifungal activities against phytopathogen Colletotrichum, causing agent of chili anthracnose disease.
Chili is an essential horticultural commodity in tropical and subtropical countries, including Indonesia. Indonesia together with other Asian countries such as Thailand, China, Turkey, and India are known as the largest chili producers in the world (Saxena et al., 2016). However, there are obstacles in chili production, especially anthracnose disease caused by Colletotrichum sp. (De Silva et al., 2017). This fungus causes the black spots on the surface of the chili fruit, thus reducing its marketability (Than et al., 2008). Chili Anthracnose disease causes significant yield losses globally; for example, in Thailand, yield
losses of up to 80% (Montri et al., 2009), 10% in Korea (Kim et al., 2008), about 10-80% and 2-35% in the rainy season and dry season respectively in Indonesia (Than et al., 2008). To control this disease, we investigate the antifungal activity of bacteria isolated from frog skin against Colletotrichum. This study is the first report using frog skin bacteria as a potential biological control agent.
MATERIALS AND METHODS
Microorganisms and culture conditions
Five bacterial isolates from Indonesian’s frog skin species Fejervarya limnocharis namely KSMD3; KSMD9; KSMD10; KSMV12; KSMV15 were used in this study. Pure bacterial cultures stored at –80ºC in 10% skim milk were revived on NA agar plates and incubated at 28ºC for 48 h. Colletotrichum capsici TCKr2, the chili anthracnose fungus was used. The fungi was cultured on PDA (potato dextrose agar) medium at 28°C in the dark (Kubota and Abiko, 2000). Ten-day old PDA plate cultures of C. capsici TCKr2 was flooded with 10 ml sterile distilled water and carefully scraped with a paint brush to harvest conidia. The conidial suspension was filtered through two layers of sterilized cheesecloth, and the density was adjusted to 105 conidia/ml using a haemocytometer under microscope (Olympus, Tokyo, Japan).
Primary screening of antagonistic bacteria by dual culture method
All five bacterial isolates were initially screened for their antifungal activity against the chili anthracnose pathogen C. capsici TCKr2 by the dual-culture method (Damasceno et al., 2019) with a slight modification. Briefly, a 10-day old mycelial plug of C. capsici (ø3 mm) obtained from the PDA culture was placed on the centre of plate contained a fresh NA added with 2% of dextrose (ø90-mm Petri dish) and incubated in the dark at 29ºC for 24 h. A loop full of bacteria was streaked 3.0 cm away from the fungal pathogen on plate, incubated in the dark at 29ºC for 3 days. Percent inhibition of mycelial growth (GI) was calculated as: GI (%) = [(R-r)/R] × 100; where, R represents the colony radial size (mm) of the fungus distal to the bacteria (as a control) and r represents the colony radial size (mm) proximal to the bacteria.
The alteration of fungal morphology was examined by taking a treated aerial mycelium of fungi with a sterilized needle and put on the clean slide glass. Then the fungus was stained with 0.01% (w/v) trypan blue in lactophenol dye. Fungal morphology was observed using a light microscope.
Chili anthracnose disease resistance assay
The fresh chili pepper (Capsicum frutescens L.) fruit washed with distillate sterile water and allowed to air dry. The surface of dried chili were sterilized with 70% of ethanol and wiped them with a sterile tissue paper (Rajapakse & Ranasinghe, 2002). All sterilized chili pepper, were wounded in two site (4.0mm in diameter) and dropped 3µl of bacterial suspension (1010 cfu/ml). After 2h, the 3µl (105 conidia/ml) fungal pathogen was inoculated to each wound. The fungicide mancozeb was used as a control. Then, all treated chili were kept in a transparent box for 7 days with wet filter paper inside to maintain the humidity. After incubation period, the disease incidence was measured using the formula described by Cardoso et al. (2004) 1 = Σx∕N where x represents the number of diseased plants divided by the total number of evaluated plants.
Molecular identification of bacteria using analysis 16S rDNA gene
Genomic DNA of selected bacteria was extracted using the Wizard Genomic DNA Purification Kit following the manufacturer’s instructions. Bacterial isolates were cultured in nutrient broth at 37°C for 24h. The bacterial cells were harvested by centrifugation (536 ×g) at 4°C for 15 min. An approximately 1.5-kb fragment of the bacterial 16S rRNA gene was amplified using the universal primer set 27F (5’-AGAGTTTAGTCCTGGCTCAG-3’) and 1529R (5’-CAIAAAGGAGGTGATCC-3). The total reaction volume per vial was 10.0 μl using kit MegaMix Blue (Microzone Ltd). Reactions were held at 94°Cfor 5 min to denature the DNA, with amplification proceeding for 30 cycles at 94°C for 1 min, 55°C for 1.5 min, and 72°C for 2 min, and a final extension at 72°C for 10 min. The amplicon was visualized in a 2% (w/v) agarose gel and subjected to DNA sequencing (ABI PRISM310 Genetic Analyzer, Thermo Fisher Science, Waltham, MA, USA). Primers used for sequencing were the same as those used for amplification of the 16S region (27F and 1492R).
Sequence analysis
The sequence of each isolate was analyzed for its similarity with the sequences in the NCBI GenBank database (http://www.ncbi.nlm.nih.gov) and aligned by Clustal W (Thompson et al., 1994). A phylogenetic tree was constructed with Neighbor-Joining analysis (NJ) using MEGA 11 (Kumar et al., 2018).
RESULTS AND DISCUSSION
Primary screening of bacterial frog-skin isolates for antagonistic activity against C. capsici
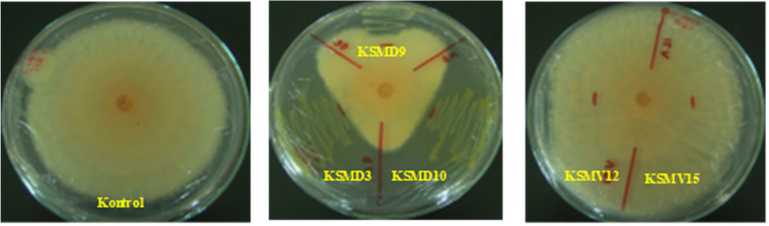
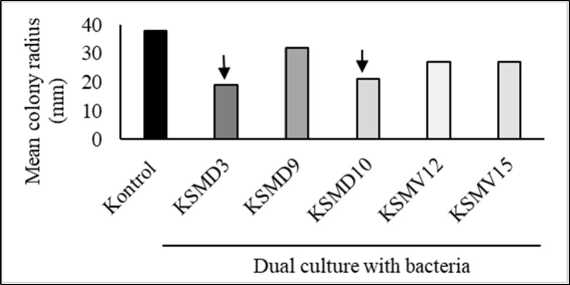
Two of the five bacteria, KSMD3 and KSMD10, showed high inhibitory activity against C. capsici (Fig. 1.A.). The percentage of mycelial growth inhibition achieved by KSMD3 was 50.0% followed by KSDM10 with 45.0% (Fig. 1.B).
Frog-skin bacteria has been widely investigated for protecting the amphibian fungal pathogen, Batrachochytridium dendrobatidis and B. salamandrivorans by producing antifungal compounds (Woodhams et al., 2018). In this study, we evaluated the efficacy of frog-skin bacteria in controlling the phytopathogen. Two selected isolates, KSMD3 and KSMD10 showed high capability inhibited the growth of C. capsici. It is indicated that these two isolates releases antifungal compound. However, the study on the mechanisms involved in this inhibition is required.
Colletotrichum has been considered as one of the most important fungal pathogen genera among other ten genera which significantly reduce the productivity (Dean et al., 2012). Application of synthetic fungicide is still popular to control the disease caused by Colletotrichum, however the excessive application can promote the fungal resistance to fungicide (Jamalizadeh et al., 2011). Thus, the application of biological control agent for instance bacteria can help to tackle the attacking of fungi.
(A)
(B)
Figure 1. Five tested frog-skin bacterial isolates, KSMD3, KSMD10, KSMD9, KSMV12 and KSMV15 streaked on PDA medium inhibited the mycelial growth of C. capsici TCkr2 in dual cultures incubated at 29°C. 3-days post inoculation of inhibited fungal pathogen viewed from above of the PDA medium (A). The radius of colony on the side nearest the bacteria was compared with the control (B). KSMD3 and KSMD10 showed the strongest inhibitory toward C. capsici.
In vitro interactions between the frog-skin bacteria and C. capsici
Treated mycelial fungal with frog-skin bacteria was observed with a light microscope. C. capsici treated with one of the isolates (KSMD3 and KSMD10) resulted in swelling and lysis of hyphae (Fig. 2).
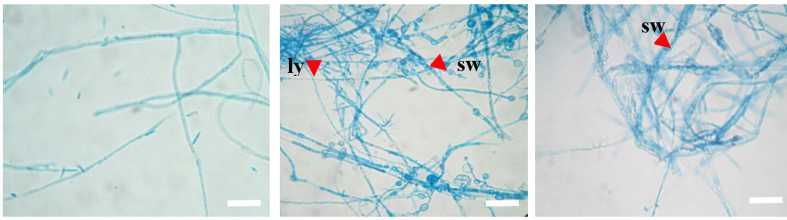
Figure 2. Treated Colletotrichum capsici TCkr2 with selected bacterial isolates KSDM3 (middle) and KSMD10 (left) after 3-days incubation at 29°C. No abnormal hyphae were observed in the control (right) without bacteria; whereas in the presence of bacteria, some abnormal mycelia formed, including swollen hyphae (sw) and lyzed (ly). Scale bars 20 µm.
The suppressive effect of two selected bacteria KSMD3 and KSMD10 presented the alteration of fungal hyphae after co-culture with C. capsici. Hyphal morphology was observed with a light microscope resulted in swelling hyphae (sw) and lysis of hypae (ly) (Fig. 2). These two bacteria indicated the induction of morphological changes. A previous study by Kwon et al., (2022) showed that
Bacillus tequilensis GYUN-300 induced the changes of mycelial Colletotrichum acutatum, where the conidia has swollen. Another study by Zepeda-Giraud et al., (2020) demonstrated the damage of C. gloesporoides mycelia after treated with biocontrol agent yeast Wickerhamomyces anomalus. Likewise, Susilawati et al., (2021) reported that three bacterial isolates frog skin causes severe changes in mycelia of C. orbiculare A-19. The mode of action involved in this mycelial alteration included the production of hydrolytic enzymes (Zepeda-Giraud et al., 2020), nutrient competition, hyperparasitism and antibiosis (Köhl et al., 2019).
Chili anthracnose disease resistance assay
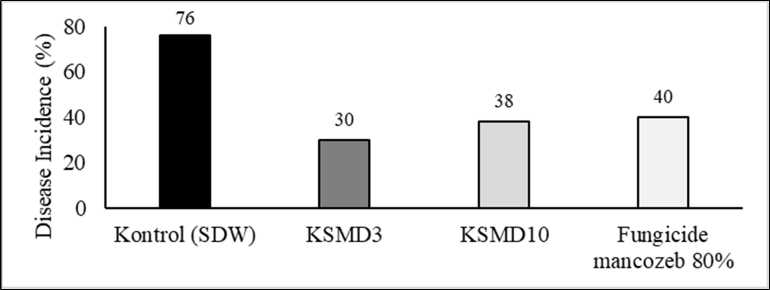
Two selected bacteria, KSDM3 and KSMD10 were tested for their resistance activity toward C. capsici using fresh chili pepper. KSMD3 showed the lowest disease incidence percentage with 30% compared to control using SDW and the commercial fungicide with 76% and 40% respectively (Fig. 3.B). The mycelial of C. capsici growing well covered whole the fruit of chili pepper after 7 days-inoculation in control treatment, while chili fruit treated with KSMD3 the mycelial fungi did not show high severity disease (Fig. 3.A). The treatment of chili with KSMD3 proved satisfactory results, with the percentage of DI (30%) lower than the control (76%) and KSMD10 by 38%. The high inhibition demonstrated by KSMD3 and KSM10 could be due to the production of antifungal substances, however the mode of action of the inhibitory activity of both isolates is still unknown. Therefore, the further study is required.

(A)
(B)
Figure 3. Two selected frog-skin bacteria KSMD3 and KSMD10 were dropped on wounded healthy chili fruit after 24h inoculated with C. capsici. controlled chili fruit with SDW showed high severity disease with chili fully covered with mycelium of C. capsici after 7-days inoculation compared to KSMD3 treatment (A). The lowest percentage of disease incidence showed by treatment with KSMD3 followed by KSMD10, while application of fungicide mancozeb 80% presented slight disease incidence compared to KSMD3 (B).
Molecular identification of best bacteria using analysis 16S rDNA gene
We sequenced the 16S rRNA of best bacteria KSMD3. The sequence was 99.28% identical to those of Pseudomonas aeruginosa (for example NR114471.1; Fig. 4). We identified KSMD3 as a Pseudomonas sp. Pseudomonas are widely known as effective biological control (Höfte, 2022). According to Lahlahi et al. (2022) genus of Bacillus and Pseudomonas are mostly important genera which high capability in secondary metabolites production. Pseudomonas has been reported able to inhibit several most important plant diseases such as P. chloroaphis zm-1 isolated from rhizosphere showed high inhibition of fungal pathogen causing peanut stem rot, Sclerotium rolfsii by producing 1-hydroxy phenazine (Liu et al., 2022), P. aeruginosa Ld-08 effectively suppressed the growth of three tested phytopathogens, Fusarium oxysporum, Botrytis cinerea, Botryosphaeria dothidea, and Fusarium fujikuroi due to its ability of several bioactive substances production such as quinolones; 3,9-Dimethoxypteroc arpan; cascaroside B; dehydroabietylamine; epiandrosterone; nocodazole; oxolinic acid; pyochelin; rhodotulic acid; 9,12-octadecadienoic acid; di-peptides; tri-peptides; pinolenic acid methyl ester; ursodiol; and venlafaxine (Khan et al., 2022). From these studies, Pseudomonas isolated from common niche of environment for instance soil, rhizosphere, or water (Lahlahi et al., 2022). In this study, we isolated Pseudomonas from amphibian skin. To date, this study is the first report of bacteria isolated from frog skin showing the antagonistic effect on Colletotrichum sp.
— NR 113599.1 Pscudomonas aeruginosa strain NBRC 12689
NR 026078.1 Pscudomonas aeruginosa strain DSM 50071
--- FJ805450.1 Pscudomonas aeruginosa strain RM3
— KSMD3.357F
— NR 114471.1 Pscudomonas aeruginosa strain ATCC 10145
--- LT745896.1 Pscudomonas aeruginosa strain D4
NR 117678,1 Pscudomonas aeruginosa strain DSM 50071
— NR 043420.1 Pscudomonas Iluorcsccns strain IAM 12022
— MN 153455.1 Pscudomonas sp. strain MS-bac-6
OQ 168635.1 Pscudomonas aeruginosa strain (>Z7
Figure 4. Neighbor joining (NJ) phylogenetic trees constructed based on 16S rRNA gene sequence indicate relationships between KSMD3 and Pseudomonas spp. Values at the nodes represent the percentage NJ bootstrap values from 1000 replicates; values ≥ 50% are shown. Bars indicate phylogenetic distances of 10%.
CONCLUSION
In conclusion, Pseudomonas sp. KSMD3 isolated from the skin of the Indonesian native frog species, F. limnocharis, is a candidate for biocontrol agents of plant disease caused by Colletotrichum capsici. The mode of action responsible for the control of disease seemed to be antibiosis, however further analysis to study this mode of action is necessary to be done.
ACKNOWLEDGEMENT
We thank MORA (Ministry of Religion Affair Republic Indonesai) for fully funding support this research. D.E. Saputro (Integrated Lab UIN Sunan Kalijaga) for his help providing all material needed. S.J.Munawwaroh, for providing fungal pathogen culture C. capsici.
REFERENCES
Abbassi F, Oury B, Blasco T, Sereno D, Bolbach G, Nicolas P, Hani K, Amiche M, Ladram A.
2008. Isolation, characterization, and molecular cloning of new temporins from the skin of the North African ranid Pelophylax saharica. Peptides 29(9): 1526–1533.
Al-Ghaferi N, Kolodziejek J, Nowotny N, Coquet L, Jouenne T, Leprince J, Vaudry H, King, Jay. D, Conlon JM. 2010. Antimicrobial peptides from the skin secretions of the SouthEast Asian frog Hylarana erythraea (Ranidae). Peptides 31(4): 548–554.
Austin R. M. 2000. Cutaneous Microbial Flora and Antibiosis in Plethodon Ventralis. In: Bruce RG, Jaeger LD. Houck (eds) The Biology of Plethodontid Salamanders, 451–462): Springer US.
Cardoso JE, Santos AA, Rossetti AG, Vidal JC. 2004. Relationship between incidence and severity of cashew gummosis in semiarid north-eastern Brazil. Plant Pathology 53(3): 363– 367.
Conlon JM. 2011. Clinical Applications of amphibian antimicrobial peptides. Journal of Medical
Sciences, 4(2): 62–72. https://doi.org/10.2174/1996327001104020062.
D’Auria FD, Casciaro B, De Angelis M, Marcocci ME, Palamara AT, Nencioni L, Mangoni, ML. 2022. Antifungal activity of the frog skin peptide Temporin G and its effect on Candida albicans virulence factors. International Journal of Molecular Sciences 23(11): 6345.
Damasceno CL, Duarte EAA, dos Santos LBPR, de Oliveira TAS, de Jesus FN, de Oliveira LM, Góes-Neto A, Soares ACF. 2019. Postharvest biocontrol of anthracnose in bananas by endophytic and soil rhizosphere bacteria associated with sisal (Agave sisalana) in Brazil. Biological Control, 137, 104016.
Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD. 2012. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Molecular Plant Pathology 13(4): 414–430.
De Silva DD, Crous PW, Ades PK, Hyde KD, Taylor PWJ. 2017. Lifestyles of Colletotrichum species and implications for plant biosecurity. Fungal Biology Reviews 31(3): 155–168.
Höfte M. 2021. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant diseases. In: Wageningen University & Research, The Netherlands & J. Köhl (Eds.), Burleigh Dodds Series in Agricultural Science (pp. 301–374). Burleigh Dodds Science Publishing.
Iskandar DT, Erdelen WR. 2006. Conservation of amphibians and reptiles in Indonesia: Issues and problems 4(1): 60-87.
Jamalizadeh M, Etebarian HR, Aminian H, Alizadeh A. 2011. A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage: A review of mechanisms of action of biological control organisms. EPPO Bulletin 41(1): 65–71.
Jared C, Mailho-Fontana PL, Marques-Porto R, Sciani JM, Pimenta DC, Brodie ED, Antoniazzi MM. 2018. Skin gland concentrations adapted to different evolutionary pressures in the head and posterior regions of the caecilian Siphonops annulatus. Scientific Reports 8(1): 3576.
Khan N, Maymon M, Hirsch A. 2017. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 5(4): 75.
Kim TJ, Sook-Young P, Woobong C, Yong-Hwan L, Heung TK. 2008. Characterization of Colletotrichum isolates causing anthracnose of pepper in Korea. Plant Pathology Journal 24(1): 17-23.
Köhl J, Kolnaar R, Ravensberg WJ. 2019. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Frontiers in Plant Science 10: 845.
Kubota M, Abiko K. 2000. Induced resistance in hypocotyl of cucumber by infection with Colletotrichum lagenarium in leaves. J Gen Plant Pathol 66: 128–131.
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35(6): 1547–1549.
Kwon HT, Lee Y, Kim J, Balaraju K, Kim HT, Jeon Y. 2022. Identification and characterization of Bacillus tequilensis GYUN-300: an antagonistic bacterium against red pepper anthracnose caused by Colletotrichum acutatum in Korea. Frontiers in Microbiology 13: 826827.
Lahlali R, Ezrari S, Radouane N, Kenfaoui J, Esmaeel Q, El Hamss H, Belabess Z, Barka EA. 2022. Biological control of plant pathogens: a global perspective. Microorganisms 10(3): 596.
Liu C, Hong J, Yang H, Wu J, Ma D, Li D, Lin D, Lai R. 2010. Frog skins keep redox homeostasis by antioxidant peptides with rapid radical scavenging ability. Free Radical Biology and Medicine 48(9): 1173–1181.
Liu F, Yang S, Xu F, Zhang Z, Lu Y, Zhang J, Wang G. 2022. Characteristics of biological control and mechanisms of Pseudomonas chlororaphis zm-1 against peanut stem rot. BMC Microbiology 22(1): 9 .
Madison JD, Berg EA, Abarca JG, Whitfield SM, Gorbatenko O, Pinto A, Kerby JL. 2017. Characterization of Batrachochytrium dendrobatidis Inhibiting bacteria from amphibian populations in Costa Rica. Frontiers in Microbiology 8: 290.
Mangoni ML, Miele R, Renda TG, Barra D, Simmaco M. 2001. The synthesis of antimicrobial peptides in the skin of Rana esculenta is stimulated by microorganisms. The FASEB Journal 15(8): 1431–1432.
McCoy KA, Peralta AL. 2018. Pesticides could alter amphibian skin microbiomes and the effects of Batrachochytrium dendrobatidis. Frontiers in Microbiology 9: 748.
Montri P, Taylor PWJ, Mongkolporn O. 2009. Pathotypes of Colletotrichum capsici, the causal agent of chili anthracnose in Thailand. Plant Disease 93(1): 17–20.
Saxena A, Raghuwanshi R, Gupta VK, Singh HB. 2016. Chilli anthracnose: the epidemiology and management. Frontiers in Microbiology 7: 1527.
Smith HK, Pasmans F, Dhaenens M, Deforce D, Bonte D, Verheyen K, Lens L, Martel A. 2018. Skin mucosome activity as an indicator of Batrachochytrium salamandrivorans susceptibility in salamanders. PLoS ONE 13(7): e0199295.
Stecher G, Tamura K, Kumar S. 2020. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Molecular Biology and Evolution 37(4): 1237–1239.
Susilawati L, Iwai N, Komatsu K, Arie T. 2021. Antifungal activity of bacteria isolated from Japanese frog skin against plant pathogenic fungi. Biological Control 153: 104498.
Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PWJ. 2008. Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathology 57(3): 562–572.
Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acid. res. 22: 4673-4680.
Varga JFA, Bui-Marinos MP, Katzenback BA. 2019. Frog skin innate immune defences: sensing and surviving pathogens. Frontiers in Immunology 9: 3128.
Woodhams DC, LaBumbard BC, Barnhart KL, Becker MH, Bletz MC, Escobar LA, Flechas SV, Forman ME, Iannetta AA, Joyce MD, Rabemananjara F, Gratwicke B, Vences M, Minbiole KPC. 2018. Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microbial Ecology 75(4): 1049–1062.
Xu X, Lai R. 2015. The chemistry and biological activities of peptides from amphibian skin secretions. Chemical Reviews 115(4): 1760–1846.
Zepeda-Giraud LF, Olicón-Hernández DR, Pardo JP, Villanueva MGA, Guerra-Sánchez G. 2020. Biological control of Thielaviopsis paradoxa and Colletotrichum gloeosporioides by the extracellular enzymes of Wickerhamomyces anomalus. Agriculture 10(8): 325.
117
Discussion and feedback