The Effect of Reduce Salinity on Behavior and Stress Response in Vannamei Shrimp (Litopenaeus vannamei)
on

Advances in Tropical Biodiversity and
Environmental Sciences
6(3): 85-89, October, 2022
e-ISSN:2622-0628
DOI: 10.24843/ATBES.2022.v06.i03.p04
Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/92350
The Effect of Reduce Salinity on Behavior and Stress Response in Vannamei Shrimp (Litopenaeus vannamei)
Edita Rum*, Yudiana Jasmanindar, and Ade Yulita Hesti Lukas
Aquaculture Study Program, Faculty of Marine Animal Husbandry and Fisheries, University of Nusa Cendana
*Corresponding author: editarum18@gmail.com
Abstract. One of the parameters that plays an important role in the success of vannamei shrimp cultivation is salinity. This study aims to determine the effect of decreasing salinity on the behavior and stress response of vannamei shrimp. The test animals used were vannamei shrimp, which were in logs with an average weight of 4.6-6.3g. This study used a completely randomized design (CRD) with different salinity reduction treatments in each maintenance medium, namely Treatment A without decreasing salinity, Treatment B decreasing salinity 2 ppt per 6 hours for 24 hours, Treatment C decreasing salinity 2 ppt per 4 hours for 24 hours, Treatment D decreased salinity 2 ppt per 2 hours for 24 hours, each treatment was repeated 3 times. Blood glucose was measured after the decrease in salinity and the last day of the study. Blood glucose collection was carried out at the fifth swimming leg on white shrimp using a 1 ml syringe that had been rinsed using Na Citrate with the aim that the blood glucose taken did not clot quickly. The results showed a decrease in salinity caused shrimp stress. The concentration of vannamei shrimp blood glucose increased (13.66 – 52.37mg/dl). Treatment D was the best glucose concentration to suppress blood glucose concentrations (13.66mg/dl). Based on the data analysis of blood glucose concentration (p<0.05) so that it was continued with the real difference test. Vannamei shrimp behavior during salinity reduction showed a response that was not different from all treatments. Salinity 6 ppt is good salinity in the process of suppressing blood glucose concentrations when changes in water salinity occur.
Keywords. Blood glucose, behavior, Litopenaeus vannamei, salinity;
-
I. INTRODUCTION
Vannamei shrimp (Litopenaeus vannamei) is a marine fishery commodity that has high economic value in both domestic and global markets [5]. Vannamei shrimp has a fairly high tolerance to fluctuations in salinity and temperature [7]. Vannamei shrimp cultivation in Indonesia has not been widely carried out in areas far from seawater sources [6]. Success in vannamei shrimp enlargement is one of the most important steps in the aquaculture chain system, which is to support the business of providing quality shrimp. The parameter that is very important in supporting the growth and survival of vannamei shrimp is salinity [2]. Vannamei shrimp that are 1-2 months old require a salt content of 15-25 ppt for optimal growth [13]. The advantages of this vannamei shrimp are high selling price, easy to cultivate and resistant to disease.
Environmental conditions can affect the growth and stress response of vannamei shrimp. Changes in salinity
in water can cause osmotic pressure that is different from the osmotic pressure in the body of aquatic organisms so that organisms must carry out an osmoregulation mechanism [12]. The inability of the shrimp to control the osmotic balance in their body can cause the shrimp to be stressed. In addition, changes in salinity can affect the homeostasis of vannamei shrimp which in turn has an impact on growth and survival. Changes in environmental conditions will also result in changes in the allocation of energy in the fish body [12].
Stress is a non-specific response in the body to the many needs due to exposure to stressors. Stress is usually considered as an effort to maintain environmental stability [10]. [4] To deal with environmental conditions that can affect physiological conditions and can cause stress, crustaceans can utilize energy from the glycolysis process which is regulated by crustacean hyperglycemic hormone (CHH) which functions to increase blood glucose levels in shrimp. Glucose levels are regulated in
the body as a negative feedback to maintain homeostasis in the body [4]. This study was conducted to determine the effect of decreasing salinity on the behavior and stress response of vannamei shrimp.
-
II. RESEARCH METHODS
-
A. Research Location
This research was conducted for 60 days at the Laboratory of the Faculty of Animal Husbandry, Marine and Fisheries, Nusa Cendana University, Kupang.
-
B. Tools and Materials
The tools used in this study include an aquarium, sirynge, glucoDr, digital scales and refractometer. The materials used in this study were 100 vannamei shrimp (Litopenaeus vannamei) with a weight of 4.6 - 6.3g, sea water, fresh water, 8% Na Citrate.
-
C. Research procedure
-
1. Preparation of Containers and Seeds
The aquariums used are 12 pieces with each size of 30 cm x 30 cm x 50 cm. First, the container is sterilized using chlorine to avoid pests and diseases that inhibit the growth of vannamei shrimp, soaked for one day and then rinsed with clean water. The aquarium was filled with seawater with a salinity of 30 ppt then aeration was installed for three days with the aim that the oxygen in the aquarium spread evenly. Vannamei shrimp seeds were obtained from the Secondary Fisheries Business School (SUPM) of Kupang which were imported from the Main Production Center for Superior Shrimp and Shellfish (BPIU2K) Karangasem, Bali. Shrimp used in this study amounted to 100 individuals weighing 4.6 – 6.3 g each. Before conducting the research, the shrimp were acclimatized in seawater with a salinity of 30 ppt for 7 days with the aim that the shrimp could adapt to the water to be used in the research container. Then put into an aquarium with a salinity of 30 ppt as many as 10 individuals per aquarium. Each container is installed with aeration in order to supply oxygen in the maintenance container.
-
2. Raising Vannamei Shrimp
Prior to the decrease in salinity, the vannamei shrimp were kept in the aquarium for three days. The decrease in salinity was carried out for 24 hours. Then reared again for 26 days. During maintenance, the shrimp were fed pellet feed with a frequency of 4 times a day. Feeding is done using a blind feeding system or is fed to the fullest. Water changes are carried out when the water is cloudy by removing 50% of the water from the aquarium. Then filled again according to the initial volume. Removal of excreta in the form of feces and leftover feed at the
bottom of the aquarium is carried out by siphoning every 4 days.
-
3. Research Design
To determine the salinity in each rearing container using fresh water with the diluent method. The method used in this study is the Completely Randomized Method (CRD) with 4 treatments and 3 replications as follows: Treatment A: no decrease in salinity (30 ppt); Treatment B: decrease in salinity 2 ppt per 6 hours for 24 hours; Treatment C: decrease in salinity 2 ppt per 4 hours for 24 hours; and Treatment D: decrease in salinity 2 ppt per 2 hours for 24 hours
-
D. Parameters observed
-
1. Behavior
Shrimp behavior is an adaptation response made to their environment. Behavioral adaptation is an activity or behavior of an animal that adapts to environmental conditions to help it survive. The behavior observed in this study was the movement of vannamei shrimp during the process of decreasing air salinity.
-
2. Blood Glucose
Blood glucose collection was carried out before the decrease in salinity and after the decrease in water salinity. Blood glucose measurement is done as an indicator of stress. Blood glucose testing was carried out on the GlucoDR device. Blood glucose collection was carried out on the fifth swimming leg of shrimp using a 1 ml syringe that had been rinsed with 8% Na Citrate so that the glucose taken did not clot quickly.
-
E. Data analysis
This research used two types of data analysis, namely analysis of variance (ANOVA) and quantitative methods. Analysis of variance (ANOVA) was used to determine the effect of treatment on the tested parameters and if the treatment had a significant effect on the tested parameters, further tests were carried out using the Honest Significant Difference (BNJ)[8]. Statistical tests were conducted with the help of SPSS software. While the descriptive quantitative method is used to analyze the data by describing or describing the data that has been collected.
-
III. RESULTS AND DISCUSSION
-
A. Behavior of Shrimp During the Water Salinity Reduction Process
The behavior of vannamei shrimp observed in this study is the movement behavior of vannamei shrimp. Vannamei shrimp culture must pay attention to water quality according to environmental conditions in order to survive. One aspect of water quality that greatly affects
the growth of vannamei shrimp is salinity. Based on the results of research conducted that when reducing salinity from 30 ppt salinity to low salinity, a decrease in salinity of 2 ppt per 2 hours, 4 hours and 6 hours vannamei shrimp movement behavior in the face of changes in salinity from the first 5-15 minutes after decreasing salinity shows different responses. In treatment B, white vannamei shrimp showed stress behavior, which was indicated by several shrimps experiencing mild stress, namely swimming up to the surface for a few minutes after which they returned to swimming slowly at the bottom of the aquarium. Treatment C showed a behavioral response which was indicated by all the shrimp swimming to the surface for the first 5 minutes and then remaining silent at the bottom of the container. Treatment D was characterized by several shrimp swimming up and down the surface of the water with very agile movements for 10 minutes. When the salinity was decreased at different times, the shrimp showed the same response.
-
B. Blood Glucose Concentration
Blood glucose is the main source of fuel supply and essential substrate for cell metabolism, especially brain cells. Figure 1 shows the results that the highest blood glucose levels occurred in treatment A, namely the maintenance of vannamei shrimp at a salinity of 30 ppt and the lowest occurred in treatment D, namely at a salinity of 6 ppt. Blood glucose of treatment A was significantly different from treatment B, C, and D. Treatment C was significantly different from Treatment A and B but not different from treatment D.
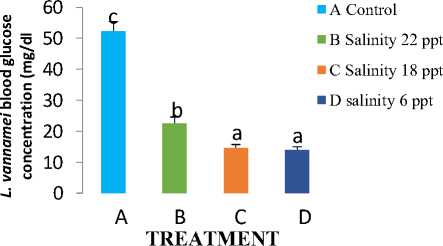
Figure 1. Increased concentration of white vannamei shrimp blood glucose during the study
Increased blood glucose concentration is considered a major indicator of stress in fish [3]. [9] stated that the presence of blood glucose is determined by stress. Hyperglycemia is an indicator of early stress, because blood glucose levels are very sensitive to stress hormones. Stress is a condition when the dynamic balance of the organism (homeostasis) is disturbed as a result of environmental factors [15]. The response to
stress is considered an adaptive mechanism of fish to maintain homeostasis [5]; [3]. When stressed, blood glucose increases in order to cope with high energy needs. If the state of high blood glucose is physiologically disturbed and can cause death [12]. Glucose is very important in meeting the high energy needs due to stress, because stress will divert energy from normal metabolic processes into energy that is used to activate physiological systems to deal with stress [1]. When stressed, the energy in the shrimp body is used more to maintain blood glucose concentrations in normal conditions so that it affects the growth and survival of vannamei shrimp.
Blood glucose is the main source of fuel supply and essential substrate for cell metabolism, especially brain cells. For the continuous functioning of the brain, glucose is needed continuously [11]. Glucose is very important to meet the high energy needs due to stress, because stress will divert energy from normal metabolic processes into energy that is used to activate physiological systems to deal with stress [1]. [17] The energy requirement to improve homeostasis during stress is met by the process of gluconeogenesis that produces glucose. Blood glucose levels are maintained homeostasis by the liver through glucose metabolism (Djauhari et al, 2019).
This study shows the range of vannamei shrimp glucose is in the normal range. [18] stated that if the glucose concentration was above 150 mg/dl, it could be indicated that the shrimp needed more energy during the molting process and in the process of maintaining homeostasis the increased glucose concentration in the blood glucose itself.
-
C. Water Quality Parameters
Water quality is one of the environmental factors that can affect the growth and survival of vannamei shrimp. The results of water quality measurements carried out for 60 days include temperature measured once a week and salinity measured once a week. The results of water quality measurements during the study can be seen in the table below:
TABLE I
WATER QUALITY DATA
|
Treatment |
Temperature (OC) |
|
A |
23-24 |
|
B |
23-25 |
|
C |
23-25 |
|
D |
23-25 |
Temperature is an indicator of physical properties parameters that are closely related to the growth and survival of white vannamei shrimp. The results of the observation that the temperature range obtained during
the study ranged from 23 - 25oC. The temperature range is included in the low range as stated by [16] that the optimal temperature range for the growth of white shrimp is 27 -30oC. However, this temperature range is still within the normal range in the cultivation of vannamei shrimp because it does not significantly affect growth, survival and dissolved oxygen in the research media. The temperature conditions in this study did not change drastically because during the study there was no significant climate change.
Salinity is a water quality factor that strongly supports the growth and survival of vannamei shrimp. Salinity which is the reference in this study is very influential on the growth of shrimp. When the change in ion exchange salinity is too high, the environmental conditions with the fluid in the cells are not balanced so that it can interfere with the metabolism and osmoregulation system of organisms (Nisa et al, 2019) so that it can cause stress.
-
IV. CONCLUSION
-
1. The behavior of vannamei shrimp during salinity reduction showed a movement response that was almost the same as all treatments.
-
2. Salinity 6 ppt is good salinity in the process of suppressing blood glucose concentrations when changes in water salinity occur.
ACKNOWLEDGMENT
The author would like to thank the Head of the Laboratory of the Faculty of Animal Husbandry, Marine and Fisheries, UNDANA who has guided and provided facilities for conducting research.
REFERENCES
-
[1] Andrade, T., A, Afonso., A, Pérez-Jiménez, O. A. Teles., V. De Las Heras., J. M. Mancera., R. Serradeiro., B. Costas. 2015. Evaluation of different stocking densities in a Senegalese sole (Solea senegalensis) farm: Implications for growth,
humoral immune parameters and oxidative status. Aquaculture 438: 6 – 11.
-
[2] Anita, A. W., M. Agus., Y. T. Mardiana. 2017. Effect of Salinity Differences on Growth and Survival of Vannamei Shrimp (Litopenaeus vannamei) PL 13. Aquaculture Study Program, Faculty of Marine and Fisheries: Pekalongan University. Aquatic Pena Journal. 16 (1):13-14.
-
[3] Barton BA. 2002. Stress in fishes: A diversity of responses with particular reference to changes ini circulating corticostreroids. Integrative and Comparative Biology 42: 517-525.
-
[4] Begg, K., N. W. Pankhurst. 2004. Endocrine and Metabolic Responses to Stress in a Laboratory Population of the Tropical Damselfish. Acanthochromis Polyacanthus. J Fish Biol 64: 133145.
-
[5] Bonga, S. E. W. 1997. The stress response in fish. Physiological Reviews 77 (3): 591-625.
-
[6] Chung, J. S., N. Zmora., H. Katayama., N. Tsutsui. 2010. Crustacean hyperglycemic hormone (CHH.) neuropeptides family : Functions, titer, and binding to target tissues. General and Comparative Endocrinology, 166 : 447-454.
-
[7] Dahlan, J., M. Hamzah., A. Kurnia. 2017. Growth of Vannamei Shrimp (Litopenaeus vannamei)
Cultured in Biofloc System with Addition of Probiotics. Fisheries Science Study Program, Faculty of Fisheries and Marine Sciences, Halu Oleo University, Kendari, Indonesia. Journal of Fishery Science and Innovation, 1(2): 1-9.
-
[8] Fitriani, N. N., Z. Abidin., M. Marzuki. 2018. Growth and Survival of Vannamei Shrimp
(Litopenaeus vannamei) in Different Salinity
Maintenance. Scientific Journal of Fisheries and Marine Affairs. Mataram University.
-
[9] Hadi, F. R., I. Riyantini., U. Subhan., Y. N. Ihsan,. 2018. Effect of Low Salinity Stress in Waters on Adaptability of Vannamei Shrimp (Litopenaeus vannamei). Padjadjaran University. Journal of
Fisheries and Marine. 9 (2) : 72-79.
-
[10] Kristiany, M. G., E. Marlina. 2014. Statistics for Aquaculture with Microsoft Excel Aquaculture Approach.
-
[11] Mazeaud, M. M., F. Mazeaud. 1981. Andrenergic responses to stress in fish, In A.D. Pickering. (Ed.). Stress and Fish. Academic Press, London., 49-75.
-
[12] McEwen, B. S. 1998. Protective and Damaging Effects of Stress Mediators. N Engl J Med., 338: 171-179.
-
[13] Nasichah Z, P. Widjanarko., A. Kurniawan., D. Arfiati. 2016. Analysis of Blood Glucose Levels of Tawes Fish (Barbonymus Gonionotus) from Rolak Songo Dam Downstream of Brantas River. Proceedings of the National Marine Seminar, Trunojoyo University, Madura, 328 -333.
-
[14] Oktari, A. D. 2017. Analysis of Glucose Levels in Windu Shrimp (Penaeus Monodon) In Pond With Immuno-Probiocirculation (Si-Pbr) System. Thesis thesis, Universitas Airlangga.
-
[15] Pamungkas, W. 2012. Osmoregulation Activity, Growth Response, and Energetic Cost in Fish Raised in a Salinity Environment. Institute of Aqua Media Fish Research and Breeding. 7 (1) :1-8.
-
[16] Rahman, F., R. Rusliadi., I. Putra. 2015. Growth And Survival Rate Of Western White Prawns (Litopaneaus vannamei) On Different Salinity.
Laboratory Aquaculture of Technology Fisheries and Marine Sciense Faculty Riau University. Jurnal Online Mahasiswa Fakultas Perikanan dan Ilmu Kelautan Universitas Riau. 9 halaman.
-
[17] Siburian, A. L. M., I. Gunawan., R. Djauhari. 2020. Calcium Phosphorus Ratio, Blood Glucose and Growth Performance of Betok Fish (Anabas
testudineus) Given Inulin Prebiotic. Aquaculture Study Program, Department of Fisheries, University of Palangka Raya. J of Tropical Anim Sci. 9(1): 1-7.
-
[18] Suprapto. 2005. Vannamei Shrimp (Litopenaeus vannamei) Cultivation Technical Instructions. CVBiotirta. Bandar Lampung. 25 halaman.
-
[19] Watkins D, Cooperstein SJ, Lazarow A. 2008. Effect of alloxan on permeability of pancreatic islet tissue in vitro. American J of Physiology. 207(2):436–440.
-
[20] Widodo, A. F., B. Pantjara., N. B. Adhiyudanto., R. Rachmansyah. 2011. Performansi Udang Vannamei (Litopenaeus vannamei) yang di Pelihara pada Media Air Tawar dengan Aplikasi Kalium. Jurnal Riset Akuakultur. 6 (2): 225-241.
Discussion and feedback