Kidney Histopathology of Wistar Rats Chronic Apical Periodontitis
on

Advances in Tropical Biodiversity and
Environmental Sciences
6(2): 50-56, June 2022
e-ISSN:2622-0628
DOI: 10.24843/ATBES.v06.i02.p04
Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/ 87381
Kidney Histopathology of Wistar Rats Chronic Apical Periodontitis that Medicated Extract Ethanol of Green Meniran Leaf
Luh Made Sudimartini1*, Anastasia Bhala2, I Wayan Wirata1, Ni Luh Eka Setiasih3, Ni Kadek Eka Widiadnyani4, I Made Merdana1
1Department of Veterinary Clinic, Faculty of Veterinary Medicine Udayana University, 2Graduated Faculty of Veterinary Medicine, Udayana University
3Histology Laboratory, Faculty of Veterinary Medicine Udayana University, 4Department of Conservation, Dentistry Education Study Program Udayana University
Jl. P. B. Sudirman, Denpasar, Bali, 80234
*Correspondent author: md_sudimartini@unud.ac.idcom
Abstract. Chronic periodontal tissue inflammation that infiltrates the periapical region and develops into a chronic lesion is known as chronic apical periodontitis. Due to the endotoxin produced by Enterococcus faecalis bacteria through damaged blood vessels in the root canal of teeth, chronic apical periodontitis damages the kidneys and increases the severity of kidney disease. The purpose of this study is to ascertain the impact of an ethanol extract of green meniran leaves on the kidney histopathology of wistar rats with chronic apical periodontitis. The material is a male wistar rat weighing between 300 and 350 grams. The four treatment groups in this study, each with 12, were separated into three subgroups according to the days 7, 14, and 21 at which the medicament paste was given. The four treatment groups in this study, each with 12, were separated into three subgroups according to the days 7, 14, and 21 at which the medicament paste was given. This study used a randomized posttest only control group design. Only E. faecalis was used to trigger the negative control group. The medication Calcium Hydroxide and Chlorhexidine Digluconate 2 percent were given to the positive control group. Treatment group 1 received Calcium Hydroxide medication including a 10 percent ethanol extract of meniran green leaves, while group 2 received Calcium Hydroxide medication along with 2 percent chlorhexidine digluconate and a 10 percent ethanol extract of green meniran leaves. Necrosis and congestion were the variables that were seen. The Kruskal-Wallis test findings revealed that the categories of congestion and necrosis of the kidneys' histopathological changes were significantly different between the K2, P1, and P2 treatments (P<0.05). The results showed that when the treatment including an ethanol extract of green meniran leaves was given to treatment groups P1 and P2, congestion and necrosis lesions significantly decreased. It is difficult to secure that giving wistar rats with chronic apical periodontitis an ethanol extract of green meniran leaves medicines can improve the histopathology.brightness.
Keywords: chronic apical periodontitis; kidney; meniran
-
I. INTRODUCTION
Inflammation of the tissues that support teeth is known as periodontitis, and it has the potential to damage the periodontal tissues. If the underlying inflammatory lesion persists for a long time into the periapical tissue and develops into a chronic lesion, periodontitis will transition to chronic apical periodontitis [1]. Enterococcus faecalis, which persists despite therapy and accounts for 85–90% of root canal infections, is one of the pathogenic microorganisms that contribute to periodontitis by being
persistent in the root canal [2]. Older cats are more likely to have periodontal disease, which is thought to increase the risk of cats and dogs developing chronic renal disease [3-4]. Systemic disease and periodontal disease interact each other. Periodontal disease can increase the risk of systemic disease, as well as systemic disease can aggravate periodontal disease [5].
Periodontitis causes kidney damage due to bacterial endotoxin infection in the bloodstream. Bacteria multiply and release endotoxins in the bloodstream, causing the
kidneys to initiate inflammatory cells as defense mediators produced by phagocytic cells, and form reactive oxygen species that cause oxidative stress and cell injury. The body has a number of physiological mechanisms in place to protect itself from damage caused by the formation of reactive oxidative species [6]. External antioxidants (exogenous antioxidants) are needed to help fight free radicals that attack the body whether they are excessive [7]. Plants can provide exogenous antioxidants, one of which is green meniran (Phyllanthus niruri Linn). Green meniran contains active compounds such as flavonoids, terpenoids, alkaloids, steroids, tannins, and saponins, according to phytochemical research [8]. Clinically, meniran herbs have antibacterial, anti-inflammatory, and antioxidant activities [9].
Previous research found that administration of meniran extract affected the microscopic appearance of the kidneys of wistar rats induced by carbon tetrachloride, with meniran extract causing fewer kidney tubules to experience degeneration and necrosis than those who were not given meniran extract. Fewer changes are due to meniran's activity as an antioxidant, which is shared by most flavonoids and works by donating hydrogen atoms to free radicals, causing these free radicals to transform into a more stable form and not cause further tissue damage. This is a cause for concern given the kidney's importance as one of the organs responsible for excretory function [10].
-
II. RESEARCH METHOD
Research Object
The objects used 48 male wistar rats, body weight range from 300-350 grams with chronic apical periodontitis. The samples taken in this study were kidney organs after chronic apical periodontitis therapy on days 7, 14 and 21.
Research Materials and Tools
The materials used in this study were wistar rat feed, 10% green meniran leaf ethanol extract, Calcium Hydroxide powder (Biodinamica, Quimica E Farmaceutica LTDA, Brazil. Imported By PT Cobra Dental, Indonesia, Yogyakarta), 2% Chlorhexidine digluconate (Cavity cleanser),Bisco Inc., Schaumburg, USA), Ketamine Hcl (Ketalar®, Warner Lambert, Ireland); Glass Ionomer Cement (GIC), ethanol 70%, 80%, 90%, 96% and absolute, cotton, aquabides, Normal Buffer Formaldehyde 10%, paraffin, Xylol, Meyer-hematoxylin, and entellan. Meanwhile, the tools used in this study included a rat cage, mixing pad, metal spatula, sample pot, gloves, scissors, tweezers, object glass, 1 ml syringe, mask, microscope and camera.
Research Design
This laboratory experimental study used a randomized posttest only control group design. 48 male Wistar rats aged 24-25 weeks with a weight range from 300-350 grams were adapted for one week. All rats were randomly divided into 4 treatment groups with three subgroups each according to the time of application of the medicament paste, so that each treatment group consisted of 12 rats. All experimental animals were conditioned to have chronic apical periodontitis by inducing E. Faecalis bacteria in the maxillary molars that had been drilled for 21 days. On day 22, all medicament treatment groups were opened with Glass Ionomer Cement (GIC) and given the prescribed medicament treatment, after being closed again using GIC to avoid contamination from outside.
The negative control group (K1) was the group that was only induced by bacteria and was patched using GIC. The positive control group (K2) was given the medicament Calcium Hydroxide and Chlorhexidine digluconate %. The treatment group (P1) was given calcium hydroxide medicament and 10% green meniran leaf ethanol extract. The treatment group (P2) was given a combination of calcium hydroxide, 2% chlorhexidine digluconate and 10% green meniran leaf ethanol extract. After being given treatment based on the specified length of time, kidney samples were taken on the 7th, 14th and 21st days, followed by the manufacture and examination of histopathological preparations. Experimental animals were sacrificed by means of ether euthanasia followed by a necropsy procedure.
Research Procedure
-
a. Preparation of experimental animals
The rats were randomly grouped into four groups, each consisting of 12 rats and then from each test group, they were divided into 3 small groups. Each group was kept in separate cages with adlibitum feeding and drinking. Male Wistar rats aged 24-25 weeks with a weight range of 300350 grams in healthy condition were acclimatized for 1 week in the experimental room. Furthermore, male wistar rats were randomized to be used as research samples.
-
b. Making green meniran leaf extract
Green meniran plants were obtained from sugarcane farmers in the Batu Malang area at the age of 3 months with a height of approximately 100 cm. The process of making simplicia powder is carried out at the UPT Laboratory of Herbal Materia Medika in Batu Malang city. The plants were then put in a greenhouse for 5 days at a temperature of 35°C-40°C. Furthermore, the selection of dry leaves by removing the stems. Then it was sifted to produce a fine powder of green meniran with a weight of ± 300 grams. Green meniran leaf simplicia is then prepared for the manufacture of green meniran leaf extract. Extracts were made using the maceration method, namely by immersing 300 grams of fine powder of green meniran leaves into a maceration vessel that was given a solvent solution of ethanol with a concentration of 96% with a
solvent volume of 1.5 liters. The immersion was allowed to stand and left at room temperature (28º-32ºC) which was protected from light for up to 3 days and was stirred regularly every day. After 3 days the filtrate was separated, the residue was then re-macerated using 1.5 liters of 96% ethanol for 3 days (accompanied by daily stirring). Then the pulp was separated by filtering which was repeated three times using gauze, so as to produce residue and filtrate. The residue is discarded and the filtrate obtained from the liquid extraction is then evaporated / concentrated using a rotary evaporator at a temperature of 700C so that the ethanol solvent evaporates and a thick extract of green meniran leaves is obtained.
-
c. Preparation of intracanal medicament paste
Calcium hydroxide Ca(OH)2 powder as much as 0.2 grams, Chlorhexidine digluconate 2% as much as 0.1 ml was prepared on a mixing pad using a metal spatula.
-
1. The positive control group used 0.2 grams of Ca(OH)2 powder and 0.1 ml of 2% Chlorhexidine digluconate. Then stirred using a cement spatula until a homogeneous consistency and produces an intracanal medicament paste with a semi-solid consistency.
-
2. The first treatment group mixed using Ca(OH)2 powder as much as 0.2 grams with 10% green meniran leaf extract as much as 0.2 ml. Then stirred using a cement spatula until a homogeneous consistency and produces an intracanal medicament paste with a thick/solid consistency.
-
3. The second treatment group mixed using 0.2 grams of Ca(OH)2 powder, 0.1 ml of 2% Chlorhexidine digluconate and 0.2 ml of 10% green meniran leaf extract. Then stirred using a cement spatula until a homogeneous consistency and produces an intracanal medicament paste with a thick/solid consistency.
-
d. Treatment
The treatment started with the rats being anesthetized first using ketamine HCl i.m with a dose of between 80 mg/kgBW with the aim of making it easier to handle the rats when opening the cavity. After the rats were completely anesthetized, the upper and lower jaws were fitted with occlusal bites to facilitate occlusal drilling of the maxillary right molars and induction of E. faecalis bacteria, then the holes were closed using GIC for 21 days so that the rats experienced chronic apical periodontitis.
The treatments were divided into 4 groups, namely: The negative control group (K1) without any treatment. The positive control group (K2) was given calcium hydroxide and 2% chlorhexidine digluconate therapy. The treatment group (P1) was given calcium hydroxide medicament therapy and 10% green meniran leaf ethanol extract. The treatment group (P2) was given a combination of calcium hydroxide, 2% chlorhexidine digluconate and 10% green meniran leaf ethanol extract. After being given treatment, it was closed again using GIC.
-
d. Sample
Sample of the kidney organs of experimental animals was carried out on days 7, 14 and 21 by euthanasia using ether. After that, the rats were subjected to a necropsy procedure using sterile surgical instruments, then the kidneys were taken and put into a sample pot containing 10% formalin. The results of kidney sampling were made into histopathological preparations for microscopic examination of the kidneys [4]. Kidney histopathology preparations were observed on a light microscope with 100x magnification for 5 different fields of view from each preparation. Subsequently, the microscopic changes found were recorded. The histopathological features of the kidneys observed included congestion and necrosis parameters. The severity of each lesion variable was scored, Score 0 for no congestion, Score 1 for focal congestion (mild), Score 2 for multifocal congestion (moderate), Score 3 for diffuse congestion (severe) and then tabulated into qualitative data.
Data analysis
Data from microscopic examination were tabulated and analyzed by Kruskal Wallis non-parametric test. If there is a significant difference (P < 0.05) followed by the Mann Whitney test. All analysis processes can be carried out with the SPSS program.
-
III. RESULTS AND DISCUSSION
Results
Descriptive analysis of the histopathological changes in kidney organs of chronic apical periodontitis wistar rats given ethanol extract of green meniran leaf revealed that there were changes in congestion and necrosis in the K2, P1 and P2 treatments, with a significant difference in the mean of K1, which was a negative control.
The results of the analysis of Kruskall Wallis on histopathological changes in the kidney organs of chronic apical periodontitis wistal rats given ethanol extract and green meniran showed that there was a significant difference in the congestion category (P < 0.05) in the K2, P1 and P2 treatments with a significant value of 0.017, respectively. 0.028 and 0.026. While in treatment K1 (negative control) there was no significant difference (P>0.05) with a significance value of 0.368. In the category of necrosis there was a significant difference (P<0.05) in the K2, P1 and P2 treatments with a significant value for the three treatmeents, 0.004. Meanwhile, in the K1 treatment (negative control) there was no significant difference in the necrosis category (P>0.05) with a significance value of 1,000. So that the Mann-Whitney analysis was continued in the treatment groups K2, P1 and P2 for the categories of congestion and necrosis.
The results of the Mann-Whitney analysis on K2, P1 and P2 treatments showed that in the congestion category in the
K2 treatment the highest congestion was on day 21, which means that in this treatment, congestion increased significantly. Where as in treatment P1, the highest congestion occurred on day 7 and decreased significantly until day 21 and in treatment P2, there was a significant decrease on day 14. While in the necrosis category, the highest necrosis in K2 treatment was on the 7th day and decreased on the 14th and 21st days. P1 treatment showed that necrosis decreased significantly on day 21. P2 treatment showed that, necrosis decreased significantly on day 14 to day 21.
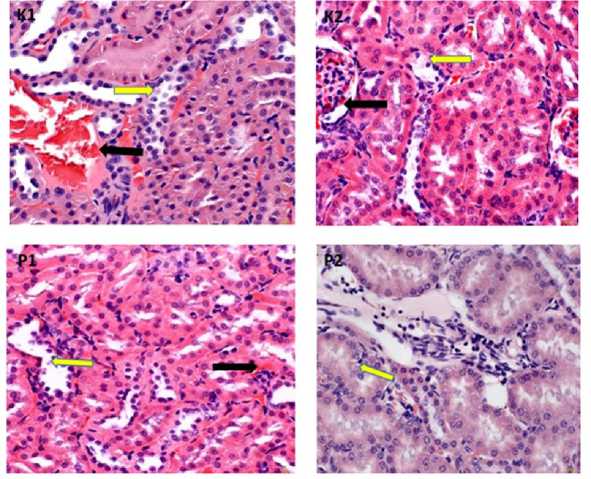
The statistical analysis above showed that the ethanol extract of green meniran leaves had an effect on the histopathological changes of the kidney of chronic apical periontitis wistar rats seen from congestion and necrosis lesions. Histopathologic of the kidneys of chronic apical periodontitis wistar rats given an ethanol extract of green meniran leaf medicament revealed improvement in congestion and necrosis lesions in Figure 1, Figure 2 and Figure 3.
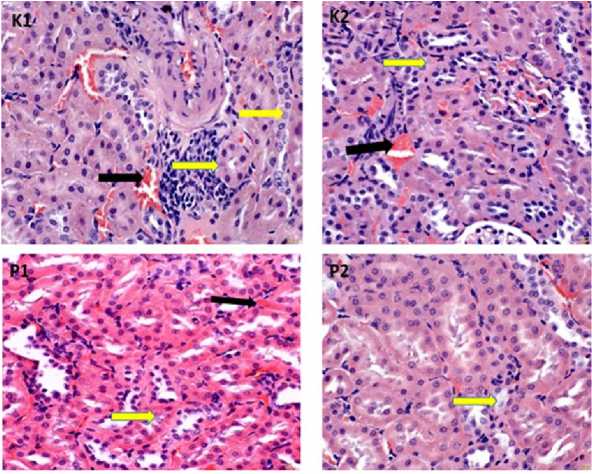
Figure 1. Histopathology of wistar rat kidney 7th day. P1= Calcium hydroxide and 10% green meniran leaf ethanol extract; P2= Calcium Hydroxide, 2% chlorhexidine digluconate, and 10% green meniran leaves ethanol extract. Congested lesion (black arrow); Necrotizing lesion (yellow arrow) (HE, 400x)
»2» V
W∙ ,. o/
Figure 2. Histopathology of wistar rat kidney 14th day. P1= Calcium hydroxide and 10% green meniran leaf ethanol extract; P2= Calcium Hydroxide, 2% chlorhexidine digluconate, and 10% green meniran leaves ethanol extract. Congested lesion (black arrow); Necrotizing lesion (yellow arrow) (HE, 400x)
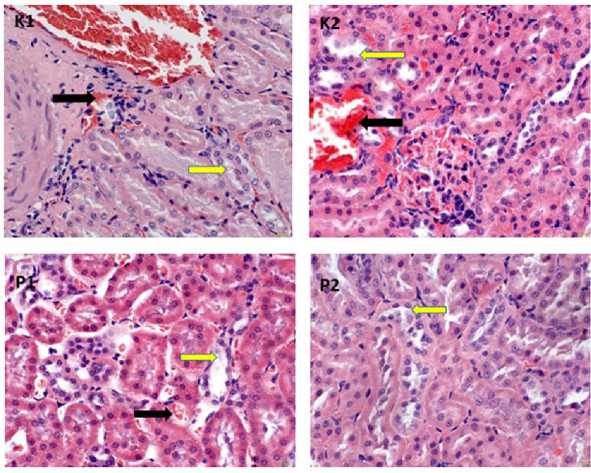
Figure 3. Histopathology of wistar rat kidney 21th day. P1= Calcium hydroxide and 10% green meniran leaf ethanol extract; P2= Calcium Hydroxide, 2% chlorhexidine digluconate, and 10% green meniran leaves ethanol extract. Congested lesion (black arrow); Necrotizing lesion (yellow arrow) (HE, 400x).
Discussion
In general, histopathological examination of the kidneys of chronic apical periodontitis wistar rats given ethanol extract of green meniran leaf revealed a significant difference (P0.05) in the presence of congestion and necrosis lesions in groups K2, P1 and P2. Congestion is a type of lesion that describes a circulatory problem. A condition in which an increase in blood flow causes an increase in blood flow to the tissues. Necrosis is the next change, which is a process of cell death caused by pathological conditions. Necrosis can be caused by infection, inflammation, or ischemia [11]
Diffuse congestion and multifocal necrosis of the tubules and glomeruli were observed in the negative control group (K1), indicating severe kidney damage. These changes are caused by the body's reaction to bacterial endotoxins that cause chronic apical periodontitis and also caused by the health status of experimental mice before being treated.
The congestion lesions were multifocal in the positive control group (K2) given calcium hydroxide and chlorhexidine digluconate as an intracanal medicament paste on the 7th and 14th days. However, the congestion of the lesion became diffuse on day 21. This is because Calcium Hydroxide, a common ingredient in pharmaceuticals, is also known to have the potential to cause side effects due to the presence of active chemicals. Meanwhile, the necrosis category was found to be multifocal on the 7th day, and focal on the 14th and 21th day. Necrosis is characterized by unclear or missing cell boundaries, cell swelling with loss of the plasma
membrane, changes in organelles, and nuclear changes under the microscope [12].
In the treatment 1 (P1), which was given Calcium Hydroxide and ethanol extract of green meniran leaves, were classified as focal congestion and necrosis. This shows there was an improvement in this treatment, but it was not significant. Because meniran is an antibacterial that plays a role in preventing kidney damage caused by bacterial endotoxin, giving ethanol extract of green meniran leaves in P1 treatment showed an improvement compared to the positive control. Meniran's antibacterial mechanism works by increasing the permeability of cell membranes, denaturing cell proteins, and damaging bacteria's cell walls and cytoplasmic membranes. Meniran, in addition to being an antibacterial, also acts as an antioxidant to repair kidney damage [13].
In treatment 2 (P2) which was given Calcium Hydroxide, 2% Klohexidine digluconate and 10% green meniran leaf ethanol extract, on the 7th day the improvement was observed to be significant which showed that the kidney histopathology was improving and getting closer to normal. Green meniran has more antioxidant power because it contains antioxidants from the outside. Green meniran has many functions, one of which is antioxidant [9]. Antioxidants are compounds that can inhibit oxidation reactions by binding to free radicals and highly reactive molecules, having allowed them to repair kidney damage caused by chronic apical periodontitis oxidative stress [14].
Based on statistical and microscopic tests, the administration of 10% green meniran leaf ethanol extract reduced and repaired kidney damage in chronic apical
periodontitis wistar rats. Meniran is one of the medicinal plants that contains numerous compounds and has been clinically tested for a variety of benefits. Green meniran phytochemicals, according to research, contain secondary metabolites of flavonoids, terpenoids, alkaloids, steroids, tannins, and saponins. Meniran has been widely reported to have antibacterial, antioxidant, anti-inflammatory, and other properties. Most flavonoids have meniran activity as an antioxidant, which works by giving hydrogen atoms to free radicals, causing these free radicals to change into a more stable form and not cause further tissue damage.
-
IV. CONCLUSION
Based on the result of this research, it can be concluded that giving an ethanol extract of green meniran leaves can improve the kidney histopathology of chronic apical periodontitis wistar rats by reducing congestion and necrosis lesions.
ACKNOWLEDGEMENT
The author would like to thank the Center for Veterinary Region VI Denpasar, Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Udayana University for assisting in the completion of this research.
REFERENCES
-
[1] Torabinejad, M., Walton, R.E. 2009. Principles and practice of endodontic. 4th ed. St. Louis. Philadelphia: Saunders Company; 2009.p. 17-20, 38-40, 58-63.
-
[2] Buck, C.G., Appelbe, O. 2006. Enterococcus Sedgley. Prevalence of Entercoccus faecalis at Multiple Oral Sites in Endodontic Patients Using Culture and PCR. Journal of Endodontic, 32(3):104-109.
-
[3] Bartlett, P. C., Van Buren, J.W., Bartlett, A. D., Chu.Z .2010. Research Article CaseControl Study of Risk Factors Associated with Feline and Canine Chronic Kidney Disease. Vet Med Int. Article ID 957570, 1-9.
-
[4] Finch, N.C., Syme, H.M. Elliott J. 2016. Risk Factors for Development of Chronic Kidney Disease in Cats. J Vet Intern Med; 30:602–610.
-
[5] Umeizu, D.K., Iwuala, S.O., Ozoh, O.B., Ekek, E.O., Umeizu, D. 2015. Periodontal Systemic Interaction: Perception, Attitudes and Practices Among Medical Doctors in Nigeria. Journal of the West African College of Surgeons.
-
[6] Purnamasari, P., Purnawati, R.D., Susilaningsih, N. 2018. Pengaruh Ekstrak Daun Sukun dan Madu Terhadap Gambaran Mikroskopik Ginjal Tikus Wistar yang Diinduksi Dietilnitrosamin. Jurnal Kedokteran Diponegoro:Vol 7, No 2, Mei 2018;1391-1405
-
[7] Kumar,V., Abbas, A.K., Aster. J.C. Buku Ajar Patologi Robbins. Trans Krisnuhoni, E. Singapore: Elsevier; 2015.
-
[8] Gunawan, I., Bawa, I., Sutrisnayanti, N. 2008. Isolasi dan identifikasi senyawa terpenoid yang aktif antibakteri pada herba meniran (Phyllanthus niruri Linn). Jurnal Kimia I2: 31-39.
-
[9] Shohaib, T., Shafique, M., Dhanya, N., Divakar, MC. 2011. Importance of Flavonoides in Therapeutics. H.J.D. Med; 3 (1): 1-18.
-
[10] Ardhini, R. 2006. Pengaruh Pemberian Ekstrak Meniran (Phyllanthus Sp.) terhadap Gambaran Mikroskopik Ginjal Tikus Wistar yang Diinduksi Karbon Tetraklorida. Semarang: Universitas
Diponegoro.
-
[11] Purwaningsih E. 2014. Pemendekan telomer dan apoptosis. Jurnal Kedokteran Yarsi 22(2): 132-141.
-
[12] Berata, I.K., Winaya, I.B.0., Adi, A.A.A.M., Adnyana, I.B.W. 2011. Patologi Veteriner Umum. Denpasar: Swasta Nulus
-
[13] Astuti, S.M. 2012. Skrining Fitokimia dan Uji Aktivitas Antibakteri Antibiotika Ekstrak Etanol Daun, Batang, Bunga dan Umbi Tanaman Binahong (Anredera cordifolia (Ten) Steenis). Pahang: Universiti Malaysia Pahang.
-
[14] Harvey, C.E., 1998. Periodontal disease in dogs:
Etiopathogenesis, prevalence, and significance. The Veterinary Clinics of North America. Small Animal Practice 28, 1111–1128.
TABLE I
KRUSKAL WALLIS ANALYSIS
|
Treatment |
Significance | |
|
Congestion |
Necrosis | |
|
K1 |
0,368ᵃ |
1.000ᵃ |
|
K2 |
0,017ᵇ |
0,004ᵇ |
|
P1 |
0,028ᵇ |
0,004ᵇ |
|
P2 |
0,026ᵇ |
0,004ᵇ |
Information: K1 = negative control (without treatment); K2 = negative control (given Calcium Hydroxide and Chlorhexidine digluconate 2%); P1 = treatment 1 (given Calcium Hydroxide and 10% green meniran leaf ethanol extract); P2 = treatment 2 (given Calcium Hydroxide, 2% Chlorhexidine digluconate, and 10% green meniran leaf ethanol extract).
TABLE II
MANN-WHITNEY ANALYSIS ON CATEGORIES OF CONGESTION AND NECROSIS
|
Treatment |
Day |
Significance | |
|
Congestion |
Nekrosis | ||
|
K2 |
7 14 |
0,317 |
0,008* |
|
21 |
0,008* |
0,008* | |
|
14 21 |
0,040* |
1,000 | |
|
P1 |
7 14 |
0,040* |
1,000 |
|
21 |
0,032* |
0,008* | |
|
14 21 |
0,127 |
0,008* | |
|
P2 |
7 14 |
0,127 |
0,008* |
|
21 |
0,127 |
0,008* | |
|
14 21 |
0,008* |
1,000 | |
Information: K1 = negative control (without treatment); K2 = negative control (given Calcium Hydroxide and Chlorhexidine digluconate 2%); P1 = treatment 1 (given Calcium Hydroxide and 10% green meniran leaf ethanol extract); P2 = treatment 2 (given Calcium Hydroxide, 2% Chlorhexidine digluconate, and 10% green meniran leaf ethanol extract).
Discussion and feedback