Malassezia sp. Infection Prevalence in Dermatitis Dogs in Badung Area
on
Advances in Tropical Biodiversity and Environmental Sciences 5(2): 45-49, August 2021 e-ISSN:2622-0628
DOI: 10.24843/ATBES.v05.i02.p02 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/72745
45
Malassezia sp. Infection Prevalence in Dermatitis Dogs in Badung Area
Putu Henrywaesa Sudipa1, Ketut Tono Pasek Gelgel1, and Putu Devi Jayanti2
1Departement of Veterinary Pathobiology, Faculty of Veterinary Medicine, Udayana University, Denpasar, Indonesia
2Departement of Veterinary Clinical Diagnose, Clinical Pathology and Radiology, Faculty of Veterinary Medicine, Udayana University, Denpasar, Indonesia Corresponding Author: henrywaesa@unud.ac.id
Abstract. Malasseziosis is a common fungal infection in dogs and it is secondary to the initial underlying dermatitis infection. These infections can worsen the prognosis of disease in dogs. The study was conducted on 26 dogs that were treated in several veterinary clinics in the Badung, Bali. This study aimed to determine the incidence rate of Malassezia sp. in dogs with dermatitis. Samples were collected using tape smear method from the skin lesion and then by microscopic examination. The results were tabulated and analyze descriptively. The results showed that 15 of the 26 samples of dogs tested positive were infected with Malassezia sp. (58%). Infection is more common in male (60%) and geriatric dogs (40%). Lesions were more common in the ear, limb, vaginal and inguinal region of infected dogs
Keywords: dog; tape smear; Malassezia sp.
-
I. INTRODUCTION
Dogs are one of the pets that are very close to humans. The benefits of raising dogs are stated to increase the owner's motivation to exercise and can reduce stress levels [1]. In addition, in almost every home in the Balinese community it is reported that they raise dogs for various purposes. However, along with its development, many health problems have been observed in dogs, especially skin health problems.
Dermatitis cases are commonly observed in Bali with various infectious agents. This is supported by the statement of Wiryana et al. [2] which states the incidence of dermatitis caused by various infectious agents in street dogs in Bali is relatively high. Dermatitis cases on stray dogs in Bali generally cause skin lesions in the form of primary, secondary, and mixed disorders [3]. One of the infectious agents of concern and can cause problem to pets and pet owners is Malassezia sp.
Malassezia sp is a fungus (yeast) which is a normal and commensal flora found in the skin layers of mammals and birds [4], [5]. Malassezia sp is classified as a dimorphic fungus since it is found in yeast and mycelial phases. This fungus is a lipophilic fungus that forms colonies in the stratum corneum of the skin [4], is oval or ellipsoidal with thick walls and without pseudomycelium production [6]. This type of leaf can be classified into 7 (seven) species. These species include Malassezia pachydermatitis, M. furfur, M. sympodialis, M. globosa, M. restricta, M. sloff and M. obtusa [7], [8]. However, Guillot and Bond [5]
reported that Malassezia sp now forms a unique cluster with 18 (eighteen) species that live on the skin and mucosa of vertebrates.
As a normal flora of the skin, Malassezia sp. generally found in limited numbers on the soles of the feet and ear canal [8], [9]. This causes Malassezia sp. can still be isolated on the skin along the external ear canal without the occurrence of external otitis infection [10], [11]. Adiyati and Pribadi [12] stated that Malassezia sp. is one of the opportunistic flora because it can cause mycosis infection in favorable conditions. However, if the conditions are favorable, there will be expansion of the lesion and infection.
Infection caused by Malassezia sp. can occur either superficial or systemic. Malassezia spp. Infection can be a primary or secondary disease. The predisposition factors are increasing in the production of sebum from the skin of infected animals or decreasing in the quality of sebum, bacterial infection of the skin, accumulation of moisture and damaging that occur simultaneously [13], [6], hypersensitivity, endocrinopathy, and the presence of keratinization disorders [9], [5].
Malassezia sp. referred to as pathogenic microorganisms in animals and humans. The transition from commensal to pathogenic can occur in dogs with certain conditions [5]. Zoonotic events occur due to direct contact of infected dogs with humans. Morris et al. [14] stated that the transfer of the infection is common from infected pet dogs to their owners.
Malassezia sp. can infect various breeds of dogs but infection is more commonly reported in Westies, shih-tzu, basset hounds, and cocker spaniels [9]. Malassezia sp. Many have been reported in pet dogs or stray dogs in Indonesia, however the data on the prevalence of Malassezia infection in Bali has not been widely reported. Observing the impact of the losses caused by infection with Malassezia sp. in dogs, this study aims to determine the prevalence of Malassezia sp. in dogs with dermatitis, especially in the Badung area, Bali.
-
II. RESEARCH METHODS
Data Collection Method
Data were collected by examining skin samples of dermatitis dogs. Samples were collected from 26 dermatitis dogs treated at the veterinary clinic in the Badung area, Bali. The examination was carried out by conducting a general examination by observing clinical symptoms in each dog. Dogs that have skin infections were subjected to a skin cytology examination using the tape smear method, a simple diagnostic method that can be done to detect Malassezia spp. [15], [5]. Data regarding sex, age, and location of lesions were recorded. Data related to age were divided into young age (≤ 12 months); adults (12-36 months); and geriatrics (≥ 36 months). The data were then described descriptively.
Tape Smear Examination
Skin cytology examination aims to determine the presence of inflammatory cells, bacterial infections or fungal infections [9]. Samples were collected using tape smear method. This technique applies transparent plaster media (tape) which was affixed twice to the part of the skin lesion that is suspected of being infected with fungi. The plaster was attached to a sterile object glass [14]. The sample was then stained using Methylene Blue [4] and observed under a microscope with 400X magnification. Through microscopic examination, identification of the infecting fungus is then carried out. If present Malassezia species such as Malassezia pachydermatis is characterized by its round to oval or classical peanut shape with monopolar budding. This lipophilic, non-lipid-dependent, non-mycelial saprophytic yeast organism is most often associated with Malassezia dermatitis (malasseziasis or Malassezia overgrowth) in dogs. Other Malassezia may uncommonly be noted as a cause of Malassezia dermatitis such as Malassezia sympodialis, which is smaller than Malassezia pachydermatis and has a more rounded bulbous shape and narrower-based monopolar budding [24]. The results of the were tabulated and explained descriptively.
-
III. RESULT AND DISCUSSION
From the results of microscopic examination of the skin cytology, 26 dermatitis dogs collected using the tape smear method, showed positive results for Malassezia sp. in 15 dogs (58%). The results of microscopic examination of the tape smear results were observed that the cell morphology was round to oval with thick walls and had hyphae with short and not straight stems (Figure 1). Microscopically, there was more than 5 Malassezia sp. so the sample dogs tested positive for Malasseziosis. Nardoni et al. [16] stated that the detection of 1 Malassezia sp. in 1 field of view showed a positive result of infection. Meanwhile, Borkar et al. [15] stated if more than 5 Malassezia sp. in the same field of view showed a positive result on the microscopic examination of the skin at 100X magnification.
Malassezia has a morphology that can be distinguished from other types of fungi. On microscopic examination, Malassezia cells will be seen in the form of round cells with thick walls, monopolar buds, having hyphae with short and not straight stems [12], [8]. Seetha et al. [4] states that Malassezia cells are round, oval, or cylindrical in diverse sizes with 1 to 8µm diameter and reproduces asexually.
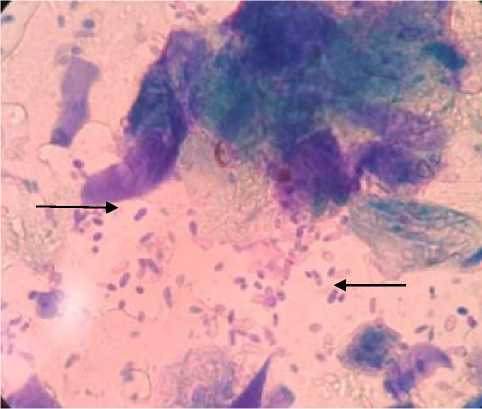
Figure 1. Microscopic examination results with 400X magnification of skin cytology samples in dermatitis dogs. The results showed positive Malassezia spp. (Black Arrows)
Based on gender, the positive results for Malassezia sp. 15 dermatitis dogs consisted of 9 males (60%) and 6 females (40%) dogs (Table 1). It can be concluded that male dogs are more at risk of infection with Malassezia sp. compared to female dogs. The same thing was reported by Seetha et al. [4] who stated that the cases of dermatitis due to Malassezia infection were higher in male dogs than female dogs. This statement is also supported by the research results of Conkova et al. [6] who stated that the
prevalence of Malassezia pachydermatis infection was relative higher in dogs of the male sex than in females.
TABEL I
MALASSEZIA SP. INFECTION IN DOGS DERMATITIS BASED ONAGE AND GENDER
|
Age |
Sex |
Total |
Percentage (%) | |
|
Male |
Female | |||
|
≤ 12 month |
3 |
1 |
4 |
27 |
|
12 – 36 month |
2 |
3 |
5 |
33 |
|
≥ 36 month |
4 |
2 |
6 |
40 |
|
Total |
9 |
6 |
15 |
100 |
|
Percentage (%) |
60 |
40 | ||
Based on the age level of the infected dog, the highest infection results were found at the age above 36 months with an incidence percentage of 40%. The prevalence of Malassezia pachydermatis infection is relatively higher in geriatric dogs than in young and adult dogs [6]. The more common infections in geriatric dogs may be attributed to the lower immunity of geriatric dogs compared to both young and adult dogs. Bond et al. [17] stated that both innate and specific immune responses play a significant role in the body's resistance to skin infections caused by fungi. Cellular immunity plays a vital role in the defense and recovery of conditions caused by exposure to Malassezia spp.
TABEL II
SKIN CYTOLOGY SAMPLING BASED ON THE DOMINANT LESION LOCATION
|
Location of Lesion |
Total |
Persentase (%) |
|
Ear |
3 |
20 |
|
Vagina & Inguinal |
3 |
20 |
|
Neck |
2 |
13 |
|
Abdomen |
1 |
7 |
|
Head |
2 |
13 |
|
Extremities |
3 |
20 |
|
Back |
1 |
7 |
|
Total |
15 |
100 |
In this study, it was observed that there were several locations where the body of the dog had dominant skin lesions including the ears, vagina and inguinal, neck, abdomen, head, extremities, and back so that cytological samples were taken at these locations (Table 2).
Lesions were more commonly observed in the ear (20%), vaginal and inguinal (20%), and extremities (20%) areas. This is in line with the statement of Nardoni et al. [18] as a potential pathogen, Malassezia spp. can be isolated from the skin around the lips, chin, anal sac, vagina, rectum, and external ear canal [19]. Seetha et al.
-
[4] stated the initial infection of Malassezia spp. seen in the abdominal area of infected animals and then spread to other body areas such as axillary and inguinal.
Parts of the body including the ears, extremities, vagina and inguinalis have a higher humidity level, which causes infectious lesions to develop more commonly in these areas than in other areas of the body. Malassezia development factors are associated with several environmental conditions, such as elevated temperature and humidity in the skin area or the location of animal rearing [6]. Matousek et al. [20] and Borkar et al. [15] stated that fungal colonies increased at high pH in certain skin areas. These results differ from reports of other researchers which state that Malassezia infection is higher in samples collected from the dorsal skin area [6] and the skin of the neck area [4]. Malassezia spp. produce keratinase enzymes and other enzymes that can digest protein complexes in the skin thus allowing these fungal species to grow deeper into the stratum corneum in animal skin and cause inflammatory reactions [4].
Clinical signs at the beginning of infection are characterized by the presence of alopecia, local and general erythema, erythematous papules and macules, crusted skin, and the presence of scale [6]. In this study, clinical symptoms were observed in sample dogs suspected of being infected with Malassezia spp. in the form of hair loss, especially in the ears, extremities and back, moderate pruritus, scale and plaque, superficial oily skin, and skin hyperpigmentation. This is in line with Coyner's [9] he mentioned dogs with malassezia spp. generally develop clinical symptoms in the form of alopecia, lichenification, and generally, oily skin accompanied by a scale. Mild to high degrees of pruritus have been observed in infected dogs [21]. This is supported by Rostaher's [11] statement that almost all dogs with Malassezia sp. will cause pruritus. Skin hyperpigmentation was observed in the ventral neck, axillary, and inguinal limbs [12]. Color changes have also been observed in the paws of infected dogs, where the skin of the claw area will be brownish red [11].
The development of clinical sign is often associated with an imbalance in the normal homeostatic immunity of the infected animal [5]. Besides other predisposing factors that can trigger infection, namely an increase in sebum production or a decrease in the quality of sebum on the skin of infected animals, bacterial infection of the skin, accumulation of moisture and skin epidermal damage, hypersensitivity, endocrinopathy, and keratinization disorders [13], [6], [9]. Malassezia sp. Infection can worsen the case of dermatitis due to other agents. Gaitanis et al. [22] and Sihelska et al. [19] stated that Malassezia sp. can participate as a cause of seborrheic dermatitis, dandruff, and folliculitis.
Malassezia sp. Infection is reported to be zoonotic from either infected animal to humans, especially animal owners if they have direct contact. Low immunity predisposes to easy transfer of infection between animals to humans. Pathological conditions in humans have been reported such as pityasis versicolar, seborrheic dermatitis, folliculitis, and systemic infections [19].
Malassezia sp. Infection can be local or general. Malassezia sp. Infection can be chronic and allows for repeated exposure so that it requires a long therapy [17]. Antifungal treatments are generally used successfully to control fungal overgrowth [23]. This in turn affects the choice of antifungal drugs to be used. Sihelska et al. [21] stated that topical antifungal drugs are preferred for local fungal infections. Whereas for more general infections it is necessary to use systemic antifungal drugs and other necessary therapies that can support increased body immunity.
-
IV. CONCLUSION
Malassezia sp. prevalence in dogs’ relative high in Badung Area with (58%), infects male dogs (60%) and geriatric dogs (40%), with lesions commonly found in the ear, limb, vaginal and inguinal areas with an incidence of 20% each. Dog age and sex predispose to the spread of Malassezia sp infection in dogs.
ACKNOWLEDGMENT
The author would like to thank his fellow veterinarians who work around the Badung area, who have been permitted to conduct research and utilize data from their patient's medical records.
REFERENCES
-
[1] Keat, K. C., Subramaniam, P., Ghazali, S. E., & Amit, N. 2016. Review on Benefits of Owning Companion Dogs among Older Adults. Mediterranean Journal of Social Sciences, 7(4): 397-397.
-
[2] Wiryana, I. K. S., Damriyasa, I. M., Dharmawan, N. S., Arnawa, K. A. A., Dianiyanti, K., & Harumna, D. 2014. Kejadian dermatosis yang tinggi pada anjing jalanan di Bali. J. Vet, 15(2): 217-220.
-
[3] Widyastuti, S. K., & Utama, I. H. 2012. Kelaianan kulit anjing jalanan pada beberapa lokasi di Bali. Buletin Veteriner Udayana, 4 (2): 81-86.
-
[4] Seetha, U., Kumar, S., Pillai, R. M., Srinivas, M. V., Antony, P. X., & Mukhopadhyay, H. K. 2018.
Malassezia Species Associated with Dermatitis in Dogs and Their Antifungal Susceptibility. Int. J. Curr. Microbiol. App. Sci, 7(6): 1994-2007.
-
[5] Guillot, J., & Bond, R. 2020. Malassezia yeasts in veterinary dermatology: an updated
overview. Frontiers in cellular and infection microbiology, 10: 79.
-
[6] Concova. E., Sesztakova, E., Palenik, L., Smrco, P., Bilek, J. 2011. Prevalence of Malassezzia pachydermatis in Dogs with Suspected Malassezia Dermatitis or Otitis in Slovakia. Acta Vet.Brno, 80: 249-254.
-
[7] Midgley, G. 2000. The lipophilic yeasts: state of the art and prospects. Sabouraudia, 38 (Supplement_1): 9-16.
-
[8] Bajwa, J. 2017. Canine Malassezia dermatitis. The Canadian Veterinary Journal, 58(10): 1119.
-
[9] Coyner, K.S. (Ed.). 2019. Clinical Atlas of Canine and Feline Dermatology. John Wiley & Sons.
-
[10] Aspres, N., & Anderson, C. 2004. Malassezia yeasts in the pathogenesis of atopic dermatitis. Australasian journal of dermatology, 45(4): 199-207.
-
[11] Rostaher, A. 2016. Malassezia Dermatitis-How do I manage this? In: 8th World Congress of Veterinary Dermatology, Bordeaux, France, 31 May 2016 – 4 June 2016.
-
[12] Adiyati, P.N., & Pribadi, E.S. 2014. Malassezia spp. dan Peranannya sebagai Penyebab Dermatitis pada Hewan Peliharaan. Jurnal Veteriner 15 (4): 570-581.
-
[13] Patterson, A.P., & Frank, L.A. 2002. How to diagnose and treat Malassezia dermatitis in
dogs. neoplasia, 1(2): 5.
-
[14] Morris, D.O., O’Shea, K., Shofer, F.S., & Rankin, S. 2005. Malassezia pachydermatis carriage in dog owners. Emerging infectious diseases, 11(1): 83.
-
[15] Borkar, R., Roy, K., Shukla, P.C., Gupta, D. 2014. Therapeutic Management of Malassezia Dermatitis in Dogs. Haryana Vet 53 (2): 106-109.
-
[16] Nardoni, S., Corozza, M., Mancianti, F. 2008. Doagnostic and Clinical Features of Animal Malasseziosis. Parasittol 4: 227-229.
-
[17] Bond, R., Patterson-Kane, J.C., & Lloyd, D. H. 2004. Clinical, histopathological and immunological effects of exposure of canine skin to Malassezia pachydermatis. Medical mycology, 42(2): 165-175.
-
[18] Nardoni, S., Dini, M., Taccini, F., & Mancianti, F. 2007. Distribusi dan ukuran populasi Malassezia pachydermatis pada kulit dan mukosa anjing atopik. Mikrobiologi veteriner , 122 (1-2): 172-177.
-
[19] Crespo, M. J., Abarca, M. L., & Cabañes, F. J. 2002. Occurrence of Malassezia spp. in the external ear canals of dogs and cats with and without otitis externa. Medical Mycology, 40(2): 115-121.
-
[20] Matousek, J. L., Campbell, K. L., Kakoma, I., Solter, P. F., & Schaeffer, D. J. 2003. Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Canadian journal of veterinary research, 67(1): 56.
-
[21] Sihelská, Z., Čonková, E., Váczi, P., & Harčárová, M. 2019. Antifungal Susceptibility of Malassezia
pachydermatis Isolates from Dogs. Folia Veterinaria, 63(2): 15-20.
-
[22] Gaitanis, G., Magiatis, P., Hantschke, M., Bassukas, ID, and Velegraki, A. 2012. Genus Malassezia pada penyakit kulit dan sistemik. Ulasan mikrobiologi klinis, 25 (1): 106-141.
-
[23] Peano, A., Johnson, E., Chiavassa, E., Tizzani, P., Guillot, J., and Pasquetti, M. 2020. Resistensi Antijamur Mengenai Malassezia pachydermatis: Di Mana Kita Sekarang? Jurnal Fungi, 6 (2): 93.
-
[24] Miller WH, Griffin CE, Campbell KL. Muller & Kirk’s Small Animal Dermatology. 7th ed. St. Louis, Missouri: Elsevier, 2013:243–249.
Discussion and feedback