Effect of Giving Different Doses of Vitamin E in Feed to the Level of Gonad Maturity of Tilapia (Oreochromis niloticus)
on
Advances in Tropical Biodiversity and Environmental Sciences 5(2): 40-44, August 2021 e-ISSN:2622-0628
DOI: 10.24843/ATBES.v05.i02.p01 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/69722
40
Effect of Thiophenone Compound on Motility and Caseinase Production of Aeromonas hydrophila
Ayu Ashari Margareth Sinaga1*, Pande Gde Sasmita Julyantotro1, and Dewa Ayu Angga Pebriani1
1Department of Aquatic Resources Management, Faculty of Marine and Fisheries, Udayana University
Jl. Kampus Unud Bukit Jimbaran, Kuta Selatan, Badung, Bali
*Corresponding author: ayuasharisng@gmail.com
Abstract. Motility and caseinase production are part of bacterial virulence factors which are regulated by a bacterial intercellular communication system, often called quorum sensing (QS). This study aims to determine the effect of the QS inhibitor compound thiophenone in reducing those virulence factors. This research was conducted at the Laboratory of Fisheries Science, Faculty of Marine Science and Fisheries, Udayana University, from December 2019 to March 2020. This study tested 3 different treatments with 3 repetitions for each treatment. Treatment A as control (without the addition of thiophenone), treatment B (addition of 5 μM thiophenone), and C treatment (addition of 10 μM thiophenone). The results showed that thiophenone compounds can inhibit the QS system of Aeromonas hydrophila. It can be seen from the significantly reduced (P<0.05) motility and caseinase enzyme activity of A. hydrophila compared to without addition of thiophenone compounds. The average diameter of the caseinase enzyme production produced in treatment A at 22 hours was 21.40±0.36 mm, in treatment B was 19.70±0.2 mm and in treatment, C was 17.87±0.05 mm. Whereas in motility, the resulting average diameter in treatment A at 22 hours was 6.57±0.61 mm, in treatment B was 5.67±0.35 mm and in treatment, C was 5.10±0.6 mm. These results indicate that the QS thiophenone inhibitor compound can reduce virulence factors, namely motility and caseinase production from pathogenic bacteria A. hydrophila. Treatment C can decrease virulence factors compared to treatment A and B.
Keywords: A. hydrophila; caseinase; motility; quorum sensing
-
I. INTRODUCTION
Aeromonas hydrophila is bacterial species that cause Motile Aeromonas Septicemia (MAS) disease in fish. In controlling MAS disease, antibiotics are still used. However, this inappropriate use of antibiotics has been led to the negative impact of the emergence of resistant pathogenic bacterial and the antibiotic residues resulted in the pollution of aquatic environment [1].
One of the virulence factors of A. hydrophila is the production of caseinase enzyme and its motility. The activity of these two virulence factors is regulated by a communication system between bacterial cells called quorum sensing (QS). Caseinase is proteolytic enzyme, which can take a nutrient supply and fight the hosts body defense, allowing disease to be developed in the host. This enzyme can also create a damage and reduce connective tissue or muscle tissue such as collagen, gelatin and can damage the host body tissues [2]. Meanwhile, motility is the ability of bacteria to move using motion tools such as flagellum [3]. Flagellum is used by bacteria to move on the surface of the liquid or soft media [4].
Quorum sensing system inhibition is one of the novel methods used to inhibit the virulence of pathogens with low risk of resistance. The QS system is a communication
system between bacterial cells using small molecules, one of which is the compound N-butanoyl-L-homoserine lactone (C4-HSL) as an autoinducer [5].
One of the QS inhibitor compounds is thiophenone. Thiophenone compound are derivative compound which extracted from red alga (Delisea pulchra) [6], [7]. This compound has been used in the inhibition of marine pathogenic bacteria such as Vibrio harveyi but not so many applied in freshwater pathogenic bacteria such as A. hydrophila, therefore it is necessary to research the effect of thiophenone compound on virulence factors, including motility and caseinase production in A. hydrophila.
-
II. METHODS
Experimental Setup
This research was experimental with three treatments and three repetitions for each treatment. Treatment A as a control (without thiophenone), treatment B (addition of 5 μM thiophenone), treatment C (adition of 10 μM thiophenone). Thiophenone were dissolved in pure ethanol at 5 μM and 10 μM and stored at -20oC. The research was conducted at the Fisheries Laboratory, Faculty of Marine Science and Fisheries, Udayana University.
Materials
The tools used in this study were petri dishes, laminar air flow, micropipette, microtip, autoclave, caliper, sterile hockey stick, incubator, test tube, measuring cup, bunsen fire, hot plate, erlenmenyer, magnetic stirrer, ose needle, microscope binocular (Olympus CX21FSI). The materials used in this study were Luria Bertani (LB) agar, skim milk agar (SMA), liquid LB, thiophenone, aquadest, gram staining solution and alcohol 70%. The bacterial isolate used in this study were pure isolate from the collection of the Fisheries Science Laboratory.
Preparation of Bacterial Growth Medium
Media LB agar was made by dissolving 40 grams in 1000 mL of sterile distilled water. The media was heated using a hot plate and homogenized with a magnetic stirrer. Then autoclaved at the temperature of 121oC for 30 minutes.
The media was made by dissolving 3,09 grams of SMA agar powder IN 60 mL of sterile distilled water, then put it in an Erlenmeyer and heated on a hot plate and stirred using a magnetic stirrer until it became a homogeneous solution and boiled being autoclaved at 121oC for 10 minutes.
Liquid LB was made by dissolving 10 gram of peptone and 5 gram of yeast extract in 1000 mL of distilled water and heated with a hot plate. The media was autoclaved at 121oC for 30 minutes. Taken and put one bacterium from the stock culture into liquid LB incubated at 30 oC for 24 hours. Then shake it manually every 4 hours.
Luria bertani agar semi solid made by dissolving as much as 0,3 % of LB agar in 1000 mL of distilled water then sterilized by using an autoclave at the temperature of 121oC for 30 minutes.
Thiophenone stock solution
Thiophenone were dissolved in pure ethanol at 5 μM and 10 μM and stored at -20oC [8]. Before being added to LB agar as a growth medium for A. hydrophila.
Caseinase activity assay
Aeromonas hydrophila isolate that have been grown in liquid LB media for 24 hours were inoculated as much as 2 μL of liquid LB on a petri dish containing LB agar with a spot in the middle using a 2 μL micropipette then incubated at 37oC for 24 hours. Each was done in three repetitions. The production of caseinase enzymes can be seen by the diameter of the caseinase activity (clear zone) around the colony. The information of the clear zone is due to the proteolyticactivity of the caseinase enzyme.
Motility assay
LB semi solid was made by dissolving 0.3% or 0.12 gram in 1000 mL of distilled water. Then sterilized by using an autoclave at a temperature of 121oC for 30
minutes. Before being poured into the petri dish, thiophenone was added to the medium. The petri dish was filled with 20 mL of media. The bacteria were spotted in the middle using a 2 micropipette. The media that had been inoculated with the bacteria were then incubated at 28oC for 24 hours with the face up or not upside down to avoid damage to the media. Bacterial motility was characterized by the diameter of the motility around the colony. Motility diameter was measured every 2 hours for 24 hours using a caliper.
Data Analysis
Research data was analyzed using using One way Analisis of Variance (ANOVA) followed by the 5% Least Significant Different (LST) to determine the difference between hourly treatments.
-
III. RESULTS AND DISCUSSIONS
Identification and Characteristic of A. hydrophila
The results of bacterial re-cultured on LB agar media and incubated at 28oC for 24 hours were round with yellowish white color (Fig. 1).
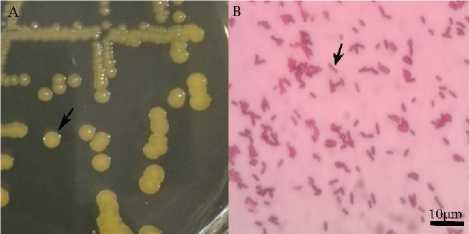
Figure 1A. Coloni A. hydrophila 1B. Chat gram A. hydrophila
-
A. hydrophila isolate used in this study is a pure isolate from the Fisheries Science Laboratory collection of Marine and Fisheries Faculty of, Udayana University. To reconfirm the cultured bacteria, gram stain method was performed. The results showed that the shape of the bacteria has red morphological characteristics, which is a gram negative bacteria (Fig. 1B). The results of bacterial identification showed that the bacteria were in accordance with the colony characteristics of A. hydrophila, namely cream color, convex elevation and smooth edges, rodshaped cell morphology and gram-negative.
Inhibitions of Caseinase Production by Thiophenone Compounds
Caseinase test was performed on skim agar (SMA) media. The result showed that A. hydrophila isolate were proteolytic, characterized by the formation of a clear zone around the bacterial colony or in the middle of the media (Fig. 2) Meanwhile, microorganisms that do not have
proteolytic or caseinase activity will not produce a clear zone or will remain cloudy around the colony [9].
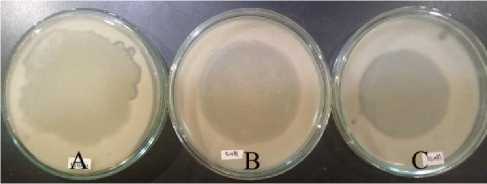
Figure 2. Diameter caseinase production. (a) Treatment A (b) Treatment B; (c) treatment C.
TABLE I
AVERAGE DIAMETER OF CLEARING ZONE OF CASEINASE
ASSAY
|
Treatment |
Average ± Standard Deviation (mm) |
|
A |
21.40 ± 0.36 |
|
B |
19.70 ± 0.2 |
|
C |
17.87 ± 0.05 |
The average clearing zone diameter of caseinase production is shown in Table I. Clearing zone diameter the diameter of caseinase production within 22 hours’ observation in treatment A was 21.40 ± 0.36 mm, treatment B 19.70 ± 0.2 mm, treatment C 17.87 ± 0.05 mm.
The first 4-hour during the caseinase production assay showed that there was no clear zone produced either in treatments A, B and C. This might because the bacteria were still in the process of enlarging cell size, increasing metabolism and adjusting to new conditions. The results of the variance test the first hour phase; 2-4 hours shows the results that are not significantly different meaning that the administration of thiophenone compounds has no significant effect at 2-4 hours on the caseinase production of bacteria A. hydrophila. This is presumably because the bacteria are still in the adaptation stage to the media and the environment. However, after 6 hours the clearing zone started to be appeared indicated that the administration of thiophenone compound had an effect on the caseinase production of A. hydrophila bacteria. Meanwhile, at the 10th hour the results were not significantly different (F count < F 5%). This is presumably because the temperature changed at the time of measuring the caseinase diameter.
A. hydrophila caseinase enzyme is controlled by the QS system with C4-HSL signaling molecules [10]. Inhibition of the QS system affect the production of the caseinase enzyme to decrease, resulting in reduced pathogenicity of A. hydrophila bacteria. Inhibition of the QS system is a new method used to prevent diseases caused by bacteria without having to inhibit the growth of these bacteria [11]. According to [12], the prevention of disease caused by bacteria by inhibiting the QS system can reduce bacterial resistance to antimicrobial substances.
The results showed that the thiophenone compound was able to inhibit the production of the caseinase enzyme from A. hydrophila bacteria. In this study, the addition of 10 μM thiophenone concentration resulted in the smallest caseinase enzyme activity. The inhibition of A. hydrophila caseinase production was indicated by the absence of a clear zone around the colony in line with the increasing concentration. If the QS system is inhibited, the production of the caseinase enzyme A. hydrophila decreases so that the hydrolyzed casein will also decrease and the clear zone diameter decreases and even does not form again [13].
According to [14], the QS system can be used as a target for inhibition of chemotherapeutic compounds. Research by [15] showed that Thiophenone was more active than brominated furanone at a concentration of 2.5 μM which had the same effect as ∼100 μM of furanone. Other factors that affect the activity of the caseinase enzyme are temperature, pH, type of substrate or media, enzyme activators and inhibitors. An increase in temperature will reduce enzyme activity and can lead to denaturation [16].
Inhibition of Bacterial Motility with Thiophenone Compound
The motility test in this research showed that A. hydrophila isolate grown in LB medium to be semi solid, experience motility or movement. This can be seen from the formation of amotility zone around the bacterial colony. The motility zone can be seen in
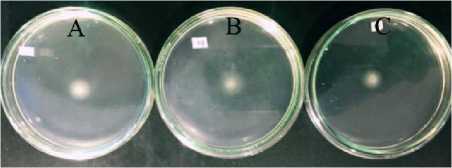
Figure 3. Diameter motility (a) Treatment A (b) Treatment B; (c) treatment C.
TABLE II
AVERAGE DIAMETER OF MOTILITY
|
Treatment |
Average ± Standar Deviation (mm) |
|
A |
6.57 ± 0.61 |
|
B |
5.67 ± 0.35 |
|
C |
5.10 ± 0.6 |
The average of motility diameter results is shown in Table 4.3. In treatment A, the average diameter motility was 6.57 ± 0.61 mm, treatment B was 5.67 ± 0.35 mm. And treatment C was 5.10 ± 0.6 mm.
The bacterial motility observed in this study was the type of swimming motility. The motility test in this study was performed a semi solid medium of LB in order to show that the bacteria are motile (movement) due to the presence of locomotion (flagellum). The flagellum is one of the
main structures outside the bacterial cell that causes movement (motility) in the bacterial cell [9]. Flagella can be physically removed from the cell. Cells that no longer have a flagellum are still alive and can synthesize new flagella [9].
The result showed that the thiophenone compound was able to inhibit the motility of A. hydrophila. In this study, the concentration of addition of 10 μM thiophenone resulted in the smallest motility. This is because thiophenone interferes with the activity of bacterial flagellum, decreased motility by thiophenone was due to reduced flagellum expression [4]. A. hydrophila has two different flagellum system, namely a polar flagellum for swimming motility and several lateral flagella to direct motility above the surface [17]. So it is suspected that the movement of bacteria is slow to get the nutrition or towards signal and result in disrupted communication between cells.
-
VIII. CONCLUSION
Thiophenone compounds was able to reduce the virulence factor of A. hydrophila such as bacteria motility and caseinase production.
ACKNOWLEDGEMENTS
Author would like to thank Kementerian Ristek Dikti which has provided PPA (Peningkatan Prestasi Akademik) scholarship.
REFERENCES
-
[1] Chu, W., S. Zhou., W. Zhu., and X. Zhuang. 2014. Quorum Quenching Bacteria Bacillus sp. QSI-1 Protect Zebrafish (Daniorerio) From Aeromonas hydrophila Infection. Scientific Reports. 4: 5446
-
[2] Secades, D AND J.A. Guijarro. 1999. Purification and Characterization of an Extracelluler Proteases from Fish Pathogen Yersinia ruckeri and Effect of Cultures Condition and Production. Applied and Environmental Microbiology. 65(9): 3969-3975.
-
[3] Madigan, M.T., J.M. Martinko and J. Parker. 2009. Brock’s Biology of Microorganisms.9th Ed. Prentice Hall Internasional, Inc. New Jersey.
-
[4] Witso, I.L., T. Benneche., L.K. Vestby., L.L. Nesse., J.L. Stensrud and A.A. Scheie. 2014. Thiophenone and Furanone in The Control of Escherichia coli O103:H2 Virulence. Pathogens and Disease. 70: 297306.
-
[5] Aini, N. 2006. Penurunan Produksi Enzim Eksoprotease Aeromonas Hydrophila Oleh Ekstrak Buah Tomat (Lycopersicon esculentum mill). Skripsi. Surakarta, Indonesia: Jurusan Biologi, Fakultas Matematika dan Ilmu pengetahuan Alam, Universitas Sebelas Maret.
-
[6] Hentzer, M., K. Riedel., T.B. Rasmussen., A. Heydorn., J.B. Anderson., M.R. Parsek., S.A. Rice., L. Eberl., S. Molin., N., Hoiby., S. Kjelleberg and M. Givskov. 2002. Inhibition of Quorum Sensing in Pseudomonas Aeruginosa Biofilm Bacteria by A Halogenated Furanone Compound. Microbiology. 148: 87-102.
-
[7] Kazlauskas, R.P., R. Murthy., Quin and R. Wells. 1977. A New Class of Halogenated Lactones from The Red Alga Delisea Fimbriata. Tetrahedron Letter. (1): 37-40.
-
[8] Yang, Q., A.A. Scheie., T. Benneche and T. Defoirdt. 2015. Specific Quorum Sensing-Disrupting Activity (AQSI) Of Thiophenone S and Their Therapeutic Potential. Sci. Rep. 5, 18033.
-
[9] Sudarsono, A. 2008. Isolasi dan Karakteristik Bakteri Pada Ikan Laut Dalam Spesies Ikan Gandara (Lepidocidium flavobroneum). Skripsi. Bogor,
Indonesia: Fakultas Perikanan dan Ilmu Kelautan, Institiut Pertanian Bogor.
-
[10] Swift, S., M.J. Lynch., L. Fish., D.F. Leink., J.M. Thomas., C.J.A.B. Stewart and Williams P. 1999. Quorum Sensing-Dependen Regulation and Blokade of Kaseinase Production in Aeromonas hydrophila. Infection and Iminity. 4:18-28.
-
[11] Manefield, M., T.B. Rasmussen., M. Henzter., J.B. Andersen., S.P. Teinberg., S. Kjelleberg., and M. Givskov. 2000. Halogenated Furanones Inhibit Quorum Sensing Through Accelerated Luxr Turn Over. Microbiology. 148: 1119-1127.
-
[12] Peters, L., G.M. Konig., A.D. Wright., R. Pukall., E. Stakebrandt., L. Eberl and K. Riedel. 2003. Secondary Metabolites Flustra Foliacea and Their Influence on Bacteria. Applied and Environmental Microbiology: 3469-3475.
-
[13] Novitasari, Y., A. Pangastuti and R. Rakhmawati. 2014. Penghambatan Produksi Enzim Kaseinase Pada Sistem Quorum Sensing Aeromonas hydrophila Dengan Pemberian Ekstrak Methanol Rimpang Segar dan Rimpang Kering Lengkuas (Alpinia galanga). Jurusan Biologi. 12(2): 51-61.
-
[14] Hentzer, M and M. Givskov. 2003. Pharmalogical Inhibition of Quorum Sensing for The Treatment of Chronic Bacterial Infection. Clint. Invest. 112: 13001307.
-
[15] Pande, G.S.J., F.M.I. Natrah., P. Sorgeloos., P. Bossier and T. Defoirdt. 2013. Quorum Sensing Disrupting Compounds Protect Larvae of the Giant Freshwater Prawn Macrobrachium Rosenbergii from Vibrio Harveyi Infection. Aqua. Res. 43: 406-407.
-
[16] Yusriah and N.D. Kuswytasari, 2013. Pengaruh Ph Dan Suhu Terhadap Aktivitas Protease Penicillum sp. Jurnal Sains dan Seni Pomits. 2(1): 2337-3520.
-
[17] Kirov, S.M., M. Castrisios and J.G. Shaw. 2004. Enterocyte Adhesins That Contribute to Biofilm
Aeromonas Flagella (Polar and Lateral) Are Formation on Surfaces. Infect. Immun. 72:1939-1945.
Discussion and feedback