Protection of Thiophenone in Catfish (Clarias sp.) Larvae When Challenged with Aeromonas hydrophila
on
Advances in Tropical Biodiversity and Environmental Sciences 5(1): 23-28, February 2021 e-ISSN:2622-0628
DOI: 10.24843/ATBES.2021.v05.i01.p04 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/68281
23
Protection of Thiophenone in Catfish (Clarias sp.) Larvae When Challenged with Aeromonas hydrophila
Ana Indriyanti1*, Pande Gde Sasmita Julyantoro2, and Ni Putu Putri Wijayanti3
123Department of Aquatic Resources Management, Faculty of Marine Science and Fisheries, Udayana University Jl. Kampus Unud Bukit Jimbaran, Kuta Selatan, Badung, Bali
*Corresponding author: anaindriyanti1@gmail.com
Abstract.This research aimed to determine the ability of quorum sensing inhibitor compound thiophenone to protect catfish (Clarias sp.) larvae when challenged with the pathogenic Aeromonas hydrophila. This research was conducted at Fisheries Laboratory, Faculty of Marine Science and Fisheries, Udayana University, from December 2019 to January 2020. The experiment was setting up with four treatments and three replications for each treatment. 10 larvae of catfish were maintained in aquarium 15 cm × 15 cm × 20 cm filled with 1 liter of freshwater and used aeration for oxygen supply. The treatments were treatment A (control), treatment B (addition of A. hydrophila 106 cfu/ml), treatment C (addition of thiophenone 10 µM), and treatment D (addition of A. hydrophila 106 cfu/ml and thiophenone 10 µM). The results showed that the addition of thiophenone 10 µM increased the survival of catfish larvae up to 73% when challenged with A. hydrophila. That was significantly difference (P<0,05) compare to treatment B with survival rate of 43% within 3 days of culture period. Although it was not statistically different (P>0,05), the highest absolute weight and length were found in treatment A of 0,47 g and 0,50 cm, respectively, while the lowest absolute weight and length were found in treatment B about 0,23 g and 0,17 cm, respectively. The water quality such as temperature, pH and DO were still within the range that supported the growth and survival of catfish larvae during this study.
Keywords: catfish (Clarias sp.), pathogen; quorum sensing; virulence
-
I. INTRODUCTION
Catfish (Clarias sp.) has great demand because it has a high nutritional content includes protein (17.7%), fat (4.8%), minerals (1.2%), and water (76%) as well as affordable prices [1]. This has an impact on the increasing interest of fish farmer for cultivating this species. Currently, catfish farming is facing several problems, including disease caused by bacteria, virus, fungi and other parasites. The larval phase of aquatic organism is very susceptible to disease. One of the diseases that often attacks catfish larvae is Motile Aeromonas Septicemia (MAS). Aeromonas Septicemia (MAS) caused by the bacterium Aeromonas hydrophila [3].
-
A. hydrophila bacteria are gram-negative bacteria which are pathogenic and can kill fish larvae up to 80100% within 1-2 weeks [4]. Prevention of diseases caused by bacterial infection is generally done by using antibiotics. The use of antibiotics is recently less efficient because it resulting in the emergence of antibiotic-resistant bacteria and antibiotic residues that can generated environment pollution [5]. One of the novel alternatives to overcome the infection of A. hydrophila bacteria is by inhibiting the quorum sensing system in those pathogenic bacteria [6].
Quorum sensing is a communication mechanism between bacterial cells using small signal molecules called autoinducer (AI). One of the compounds that can inhibit the quorum sensing system in bacteria is thiophenone [7]. The thiophenone compound is a compound derived from natural products obtained from the red algae extract Delisea pulchra [8]. The previous results showed that thiophenone compounds were able to increase the survival of giant prawn larvae when challenged with the pathogenic bacteria Vibrio harveyi without having a negative effect on the growth of giant prawn larvae [8]. However, the protection effect of this compound has never been tested on catfish larvae. Therefore, this study was conducted to determine whether thiophenone compounds can also protect catfish larvae from A. hydrophila infection without causing a negative effect on the growth of the catfish larvae.
-
II. RESEARCH METHODS
Technique sampling
This study used an experimental method using a completely randomized design (CRD). 10 larvae of catfish were maintained in aquarium 15 cm × 15 cm × 20 cm filled with 1 liter of freshwater and used aeration for oxygen
supply. This method used 4 treatments and 3 replications for each treatment, namely treatment A (control), treatment B (addition of bacteria A. hydrophila 106 cfu/ml), treatment C (addition of thiophenone 10 µM), and treatment D (addition of bacteria A. hydrophila 106 cfu/ml and thiophenone 10 µM). The research was conducted at the Fisheries Laboratory, Faculty of Marine Science and Fisheries, Udayana University.
Materials
The tools used in this study are petri dishes, loop needles, Erlenmeyer tubes, thermometer, scale, autoclave, aluminum foil, ruler, pH meter, aquarium, DO meter, aerator, incubation cabinet, and hot plate. The materials used in this study were bacteria A. hydrophila, Agar powder Luria Bertani (LB), catfish larvae, pellets, compounds thiophenone, aquades, and 70% alcohol.
Data Analysis
Research data was analyzed using One Way Analysis of Variance (ANOVA) and Duncan's advanced test with a significance level of 5%. The variables measured included the survival rate (SR) of catfish larvae and the absolute growth of catfish larvae, calculated by the formula [9]: L = Lt-Lo
Noted: L is the absolute length of the fish (cm), Lt is the length of the fish at the end of the study, Lo is the length of the fish at the beginning of the study.
W = Wt -Wo
Noted: W is the absolute weight of the fish (g), Wt is the weight of the fish at the end of the study, Wo is the weight of the fish at the beginning of the study.
the survival rate of catfish larvae was calculated used the formula [9]:
SR = -× 100
No
Noted: SR is fish survival (%), Nt is the number of fish at the end of maintenance, No is the number of fish at the beginning of stocking.
Water Quality Parameters
Measured water quality parameters include temperature, degree of acidity (pH), and dissolved oxygen (DO). These parameters were measured 2 times a day in the morning and evening during the maintenance period. Measurement of temperature, pH and DO were carried out using a thermometer, pH meter and DO meter, respectively.
-
III. RESULT AND DISCUSSION
Re-cultured of A. hydrophila
The A. hydrophila used in this study is pure isolate collection of Fisheries Science Laboratory of the Faculty
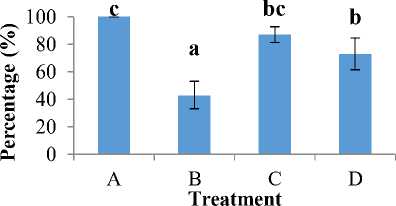
Figure 2. Survival Rate of catfish larvae
of Marine and Fisheries, University of Udayana. The results of re-culture of bacterial isolates A. hydrophila are cream to yellowish in color with smooth edges. The results of the gram stain test using a microscope with a magnification of 1000 × showed the results of the bacteria in red colour with the morphology in the form of a short rod. It indicated that the bacteria tested were A. hydrophila with gram negative because the final result of the staining was red. Colony morphology of A. hydrophila is white to yellow, convex elevation, and smooth edges, whereas cell morphology is rod-shaped and gram negative [10]. The profile of bacteria colonies A. hydrophila on agar media (A) and after gram staining (B) can be seen in Figure 1
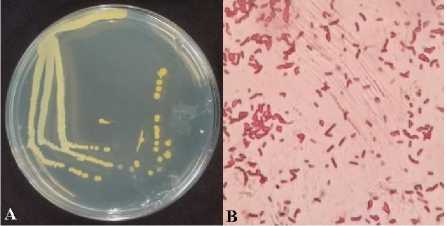
Figure 1. A. hydrophila
Survival Rate
The results showed that A. hydrophila can infect catfish larvae with a survival rate of 50% (LD50) within a period of 3 day (72 hours). The results of the highest survival rate of catfish larvae were obtained in treatment A with a percentage of 100%. Meanwhile, the lowest survival rate of catfish larvae was obtained in treatment B by 43%. Treatment C obtained a survival rate of catfish larvae of 87% and treatment D obtained a survival rate of 73%. The percentage of survival rate of catfish larvae from each treatment can be seen in Figure 2.
The results showed that the thiophenone compound is able to increase the survival rate of catfish larvae when were challenged with A. hydrophila. Catfish larvae show
clinical symptoms such as the appearance of red spots on the surface of the body and mouth. Fish larvae exposed to bacteria A. hydrophila will show red spots on the surface of the body and the most severe condition results ingnarled tail fin and severe wounds (ulcers) [11]. The compound thiophenone was then tested in vivo against catfish larvae which were infected with bacteria A. hydrophila by immersion method. The concentration of compounds thiophenone used is a compound with a concentration of 10 µM. The compound is thiophenone 10 µM used to help increase the survival of catfish larvae by disrupting the system Quorum Sensing in A. hydrophila thereby reducing its virulence. This is the same as statement [8] which proves that this compound is able to protect giant prawn larvae from bacteria Vibrio harveyi by disrupting the QS system without any negative effects on the growth and survival of larvae.
Based on the results of research that has been carried out with several treatments, the highest survival rate of catfish larvae was found in treatment A (control) which was 100% and the second highest survival rate was found in treatment C (with the addition of compound thiophenone 10 µM) which was 87%. Meanwhile, the lowest survival rate was found in treatment B (with the addition of A. hydrophila 106 cfu/ml) which was 43%. A. hydrophila are pathogenic, virulent and cause the death of 50% of the tested fish at a minimum bacterial density of 106 cfu/ml [12].
Furthermore, the results of treatment D (with the addition of A. hydrophila 106 cfu/ml and compound thiophenone 10 µM) showed that the survival rate of catfish larvae was 73%. This value indicates the effect of giving compounds thiophenone against bacterial infection A. hydrophila in catfish larvae. These results prove that the presence of an inhibitor that is able or inhibits the system QS, resulting in decreased virulence of bacteria A. hydrophila against catfish larvae. The use of compounds thiophenone as QS inhibitors is very effective in reducing the virulence of a pathogen [7]. Based on the results of the analysis carried out using ANOVA, significant results were obtained (p <0.05), where these results indicated that treatment with the addition of bacteria A. hydrophila had a significantly different effect on the survival rate of catfish larvae.
Absolute Growth
Observation results of the absolute weight gain of catfish larvae during the challenge test obtained the highest results, namely treatment A of 0.47 g and treatment B obtained the lowest yield of 0.23 g. The absolute weight gain in treatment D and C obtained results of 0.37 g and 0.30 g, respectively. The results of observations of the weight gain and absolute length of catfish larvae during the
challenge test can be seen in Figure 3 and Figure 4. The results of the ANOVA statistical test with the Duncan advanced test showed that the results between treatments did not have a significant effect on the weight gain of catfish larvae.
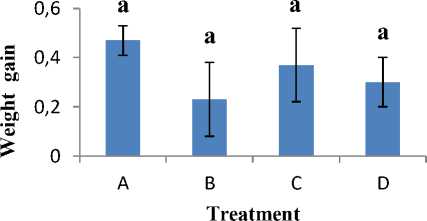
Figure 3. Absolute growth
The observations of the absolute length increase of catfish larvae show that the highest absolute length gain is in treatment A with an average of 0.50 cm and followed by treatment C of 0.37 cm. The lowest absolute length gain was found in treatment B which was 0.17 cm, while for treatment D was 0.30 cm. Based on the results of the ANOVA statistical test with Duncan's continued test, it showed that (P> 0.05), which means that each treatment had a significantly different effect on the absolute length increase of catfish larvae.
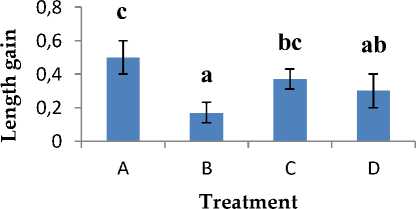
Figure 4. Absolute growth
The results of the average weight gain and length of catfish larvae can be seen in Appendix 5. The average absolute weight gain of catfish larvae ranges from 0.230.47 g. As for the increase in absolute length of catfish larvae, it has an average of 0.17-0.50 cm. The increase in weight and absolute length of catfish larvae is influenced by external factors that cause a decrease in appetite. The growth of an organism can be influenced by internal and external factors, while internal factors include heredity, resistance to disease and the ability to use food, while external factors include physical, chemical, and biological characteristics of water and food [13].
The results of these observations indicate that treatment A has a value-added weight and length which is more optimal than treatment B, C, and D. The growth of catfish larvae in treatment B has a low weight gain and length, namely 0.23 g and 0, 17 cm. This is thought to be due to the lack of appetite for catfish larvae due to infection with A. hydrophila so that growth is stunted. Fish larvae infected with bacteria A. hydrophila will experience decreased appetite [14]. The increase in weight and length of catfish larvae in treatment C and D increased which was not much different, but the two treatments had a much smaller value than treatment A. This indicated that the addition of compound gain thiophenone 10 µM in catfish larvae did not affect the weight and absolute length significantly. The use of the compound thiophenone 10 µM only specifically protects catfish larvae from infection by pathogenic bacteria by disrupting the QS system so that it does not interfere with the growth of catfish larvae. Disrupting the QS system is one way to make bacteria A. hydrophila the virulent nonvirulent by inhibiting the activity of signal molecules (C4-HSL) optimally in A. hydrophila without disturbing the growth of other organisms [15].
Water quality
This water quality measurement is carried out to control water quality during the study in order to keep it optimal and ensure that catfish larvae do not experience death due to the environment and poor water quality. Water quality parameters observed include temperature, pH, and DO Based on the results of water quality observations, the results are shown in Table 1. Based on the results of water quality observations during the challenge test, the parameter values obtained are in the optimal range for catfish larvae, namely the temperature range between 26-30oC, pH 5.6-6.6, and DO at 3.3-5.5 mg/L [20].
The results of the measurement of water quality parameters during the observation period of catfish larvae in vivo are in the optimal range, so that overall, they do not affect the mortality of catfish larvae. These results are in accordance with SNI 6484.4: 2014 which states that for the maintenance of catfish larvae, the water quality parameters are temperature ranges from 26-30oC, pH 5,6-6,6, and DO at 3,3-5,5 mg/L. So that overall, the death of catfish larvae is not caused by water quality but is caused by the addition of A. hydrophila.
The temperature values obtained during observations are in vivo the range of 26-30°C. The range of temperature values can be classified as normal temperatures for the life of cultured catfish larvae. According to SNI 6484: 2014 the water quality requirements for catfish larvae culture for temperature values that range from 25-30oC. Temperature
is a parameter of water quality that greatly affects cultivation activities because if the temperature value is below 21oC it can endanger catfish larvae such as decreased appetite eating, susceptibility to disease can even cause death [16]. Meanwhile, temperatures above 35oC will make fish larvae stress and have difficulty breathing due to increased oxygen consumption, while the solubility of oxygen in water decreases [17].
The pH value measurements during observations in vivo ranged from 5.6-6.9. From these results indicate that the pH value at the time of observation is slightly acidic because the optimal pH value in the rearing activity of catfish larvae according to SNI 6484: 2014 is in the range of 6.5-8. The pH value in treatment C and D was lower than treatment A and B, this happened because in treatment C and D the addition of compound was thiophenone given so that it had a lower pH value. Compounds Thiophenone are compounds that react with tial and amines to form amino acids and acidic [18]. However, the pH value obtained can still be said to be optimal because it does not have much difference from the pH value it should be. This is in accordance with Dahril's statement [19], that fish can still live at least at pH 4 and will die at a pH above 11.
TABLE I
AVERAGE VALUE OF WATER QUALITY PARAMETERS DURING THE STUDY
|
Parameter |
Treatment Challenge Test | |||
|
A |
B |
C |
D | |
|
Temperature (oC) |
26.9-30 |
26-29 |
26.9-29 |
26-30 |
|
pH |
6-6.9 |
5.7-6.6 |
5.6-6.3 |
5.8-6.6 |
|
DO (mg/L) |
4.8-5.5 |
3.5-4.0 |
3.3-4.0 |
4.2-5.4 |
The DO content in this observation is supplied directly by aeration, where during the research the DO value is controlled and remains in a state of control. optimal, which is in the range of 3.3-5.5 mg/L. Most fish require a DO content of 3-5 mg/L [20]. Treatments C and D had a lower DO content value compared to treatments A and B, this happened because treatments C and D had a low pH value. The DO content in the waters has decreased so the pH value will decrease because the activity of decomposing organic matter in the waters will increase CO2 so that the waters become acidic [21].
-
IV. CONCLUSION
It can be concluded that the administration of thiophenone compound 10 µM able to increase the survival of catfish larvae when challenged with pathogenic
Aeromonas hydrophila. The use of the compound thiophenone 10 µM was not significantly affect the growth of catfish larvae and has no effect on culture water quality.
ACKNOWLEDGE
Author would like to thank Allah SWT for all his blessings. Kemenristekdikti which has provided PPA scholarships as well as both parents and families who always pray.
REFERENCES
-
[1] Ubaidillah, A. & Hersulistyorini, W. 2010. Protein levels and organoleptic properties of crab nuggets with catfish (Clarias gariepinus) substitution. Jurnal Pangan dan Gizi, 1(2): 45-54.
-
[2] Elpawati, E., Pratiwi, D. R., & Radiastuti, N. 2015. Application of effective microorganism 10(EM10) for thr growth of sangkuriang catfish (Clarias gariepinus var. Sangkuriang) in the Jombang cathfish cultivation pond, Tangerang. Al-Kauniyah: Jurnal Biologi, 8(1): 6-14.
-
[3] Setyaningsih, L. 2017. Control of Aeromonas hydrophila infection in catfish with probiotic microcapsules at different doses and frequencies. Tesis. Bogor, Indonesia: Institut Pertanian Bogor.
-
[4] Cipriano, R.C. 2001. Aeromonas hydrophila and Motil Aeromonas Septicemia of Fish. United States Departement of the Interior Fish and Wild Life Service Division of Fisheries Research: Washington DC (pp.25).
-
[5] Maisyaroh, L. A., Susilowati, T., Haditomo, A. H. C., Yuniarti, T., & Basuki, F. (2018). Use of mangosteen (Gracinia mangostana) rind extract as antibacterial to treat Aeromonas hydrophila infection in tilapia (Oreochromis niloticus). Sains Akuakultur Tropis, 2(2): 36-43.
-
[6] Patel, B., Kumari, S., Banerjee, R., Samanta, M., & Das, S. 2017. Disruption of the quorum sensing regulated pathogenic traits of the biofilm-forming fish pathogen Aeromonas hydrophila by tannic acid, a potent quorum quencher. Biofouling, 33(7): 580-590.
-
[7] Witso, I. L., Benneche, T., Vestby, L. K., Nesse, L. L., Lönn-Stensrud, J., & Scheie, A. A. 2014.
Thiophenone and furanone in control of Escherichia coli O103: H2 virulence. Pathogens and disease, 70(3): 297-306.
-
[8] Pande, G. S. J., Scheie, A. A., Benneche, T., Wille, M., Sorgeloos, P., Bossier, P., & Defoirdt, T. 2013. Quorum sensing-disrupting compounds protect larvae of the giant freshwater prawn Macrobrachium rosenbergii from Vibrio harveyi infection. Aquaculture, 406(1): 121-124.
-
[9] Effendi, Bugri I.N.J., & Widanarni. 2006. Effect of density on the survival and growth of Osphronemus gourami gourami seed size 2cm. Jurnal Akuakultur Indonesia, 5(2): 127-135.
-
[10] Wahjuningrum, D., Astrini, R., & Setiawati, M. 2013. Prevention of Aeromonas hydrophila on catfish juvenile using garlic and shatterstone herb. Jurnal Akuakultur Indonesia, 12(1): 86-94.
-
[11] Nurfaidah, S. (2015). Imunogenisitas Aeromonas hydrophila Strain GK–01 DAN GB–01 Terhadap Lele Dumbo (Clarias gariepinus). Skripsi. Purwokerto, Indonesia: Universitas Muhammadiyah Purwokerto.
-
[12] Defoirdt, T. & Sorgeloos, P. 2012. Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. The ISME journal, 6(12): 2314-2319.
-
[13] Hidayat, D. & Sasanti, A. D. 2013. Survival, growth and feed efficiency of snakehead fish (Channa striata) fed raw snail flour (Pomacea sp.). Jurnal Akuakultur Rawa Indonesia, 1(2): 161-172.
-
[14] Asniatih, M. I. & Sabilu, K. 2013. Histopathological study of African catfish (Clarias gariepinus) infected with Aeromonas hydrophila bacteria. Jurnal Mina Laut Indonesia, 3(12): 13-21.
-
[15] Novitasari Y, A. Pangastuti, R. Rakhmawati. 2014.Inhibition of exoprotase enzyme production in the quorum sensing system Aeromonas hydrophila by givin methanol extract of fresh rhizome and dried galangal rhizome (Alpinia galangal). Jurnal
Biofarma, 12(2): 51-61
-
[16] Rahayu, W. 2020. Analysis of revenue of red tilapia (Oreochromis sp.) rearing in wet water ponds in polanharjo district, klaten regency. Jurnal Ilmu-Ilmu Pertanian, 7(1): 14.
-
[17] Siegers, W. H., Prayitno, Y., & Sari, A. 2019. Effect of water quality on growth of Nirwana Tilapia (Oreochromis sp.) in brackish ponds. The Journal of Fisheries Development, 3(2): 95-104.
-
[18] Benneche T, Chamgordani E.J, Scheie A.A. 2013. Reaction of (Z)-5-(Bromomethylene) thiophen-2 (5 H)-one with Some Nucleophiles in Search for New Biofilm Inhibitors. Synthetic communications, 43(3): 431-437.
-
[19] Dahril, I., Tang, U. M., & Putra, I. 2017. Effect of different salinity on growth and livelihoods of red tilapia (Oreochromis sp.) seeds. Berkala Perikanan Terubuk, 45(3): 67-75.
-
[20] Listyani. 2017.The effect of giving tempe dregs on feed on the growth of sangkuriang catfish (Clarias gariepinus). [Skripsi]. Lampung: Universitas Islam Negeri Raden Intan.
-
[21] Sinaga, E. L. R., Muhtadi, A., & Bakti, D. 2016. Vertical profile of temperature, dissolved oxygen, and
pH for 24 hours in kelapa gading lake, asahan regency, north sumatera. Omni-Akuatika, 12(2).
Discussion and feedback