THE AEROBIC EXERCISE INCREASES COGNITIVE FUNCTION BIOMARKER, KLOTHO PROTEIN, IN ALZHEIMER DISEASE
on
Sport and Fitness Journal
Volume 10, No.2, May 2022: 111-123
E-ISSN: 2654-9182
AEROBIC EXERCISE INCREASES COGNITIVE FUNCTION BIOMARKER, KLOTHO PROTEIN, IN ALZHEIMER DISEASE
Fransisca Natalia1,*, Melisa Ayu1, Kaisa Lana Afida1, Emilia Eka Arrianti1, Lina Meylyn Shine1, Nila Wahyuni2, I Putu Gede Adiatmika2
-
1 Magister Program of Biomedical Science Anti Aging Medicine Medical Faculty Universitas Udayana, 80234, Denpasar, Indonesia
-
2Physiology Department Medical Faculty Universitas Udayana, 80234, Denpasar, Indonesia
Email: fransisca.natalia_yin@yahoo.com
ABSTRACT
Aging is an inevitable process in human. It causes deterioration of many organs in human bodies such as the brain. The most common pathological aging condition in the brain is Alzheimer’s disease. This disease is the leading cause of deficits in short term memory, praxis, visuospatial, and executive function. It can impair cognitive function in older adults. A lot of intervention has been studied to treat Alzheimer’s disease. One of them is the aerobic exercise. Some studies find aerobic exercise increases the anti-aging protein, klotho protein. Klotho is a systemic-biomarkers that has an important role in sustaining cognitive function and brain integrity. The level of plasma klotho protein is increased by doing aerobic exercise moderately for 12 weeks. The increase of klotho protein improves memory formation and hippocampus function. In Alzheimer’s disease, klotho protein has the ability to lower the formation of neurofibrillary tangles. Hence, aerobic exercise is an essential intervention to improve cognitive function in Alzheimer’s disease patient.
Keywords : anti-aging; physical activity; dementia
INTRODUCTION
Aging is a process characterized by a decrease in organ function, which is caused by several genetic and environmental factors, and it can lead to death1. The number of people aged over 60 years is increasing internationally with a daily percentage reaching 8.5% of the population and is expected to increase 3 times by 20502.
Aging is usually associated with fraility. It is a syndrome that makes older adults vulnerable to stressors even in low pressure. Not only that, this syndrome leads to reduction of functional resilience and dysfunction of multi organ3. Frailty is not strongly related with age. However, it is still found to be increased when the age is older. It is found to be increased 4% in 65 years old adults, and increased 26% in 85 years old adults4. Alzheimer's disease is the most common disorder in humans aged 65 years and older. Its prevalence increases with age by 10% in humans 65 years and 50% in humans 85 years5.
Alzheimer's is a neurodegenerative disorder whose main symptom is dementia. Alzheimer's is characterized by accumulation of amyloid-b, filamentous intraneuronal inclusions formed via hyperphosphorylated Tau protein and synaptic disruption and lack of some neurons6. Cognitive symptoms of Alzheimer's are short-term memory, praxis and dysfunction of visuospatial and brain command processing7.
Aerobic exercise as a method that can improve the physical health and intellectual fitness of people with Alzheimer's disease. Cardio training consists of several low impact sports that humans must do frequently to stay healthy by walking, running, swimming, aquaerobics, and cycling8. Aerobic exercise is exercise that requires a lot of oxygen. At the stage of aerobic exercise, the uptake and release of oxygen into the body are equal. Aerobic oxidation of energy subtances required for exercise and must be provided by oxidizing stratch fat and protein in te body, aerobic exercise requires duration at least 30 minutes9.
Klotho protein is part of endocrine fibroblasts growth factor (FGF) with several enzymatic activites. Premature aging was affected by klotho protein deficiency. Insulin / IGF-I is an important signaling pathway in aging research. Klotho protein indicates IGF-I and insulin resistance. S-KL
inhibits IGF-I and insulin receptors by inhibiting tyrosine phosphorylation of both receptor and its downstream signaling protein. Blockade of s-KL-induced IGF-I signaling increases resistance to oxidative stress and improves immunity. Several studies have shown that athlete who were trained in aerobic s-Klotho will sigificantly increased and IGF-I levels deceased. Aerobic exercise is proven increase s-Klotho 10. Exercise will affect the body's by metabolism, glucose absorption, insulin sensitivity, calcium homeostasis, suppression of oxidative stress and inhibition of insulin IGF-1 and alter the pathways of body components TGFβ111. Several studies have shown that klotho protein will participate in controlling oxidative strain, ER stress, Golgi apparatus stress, cell proliferation, apoptosis and autophagy12. Klotho is associated with conditions influenced by the aging process13, such as cardiovascular disease, acute kidney disease, cancer and neurodegenerative disorders14. Klotho expression levels also decreased by age15.
Amyloidogenic mice have shown that overexpression of the klotho protein in brain can predispose to Alzheimer's disease, cognitive impairment, neurodegeneration and can also ameliorate the accumulation of Aβ in a mouse model to helps regulate Aβ-associated transporters and microglia transformation16. In Alzheimer disease, klotho protein helps to increase cognitive function, while klotho protein can be enhanced by aerobic exercise17.
Several previous studies have proven that Klotho protein increases neuronal plasticity and memory factors 18, while Klotho protein is a systemic biomarker that affects learning and memory 19. Reduced Klotho protein can cause premature aging. Aerobic exercise is very important to train physical function and quality of life 19. According to a study conducted by Saghiv athletes who were trained with aerobic exercise their s-klotho level increased significantly and their IGF-1 decreased compared to people who were not active in exercise 20. In this discussion, we focus more on improving cognitive function by doing aerobic exercise with a klotho protein as a biomarker in patients with Alzheimer's disease. This article aims to broaden knowledge and to give insights for further research about this topic.
METHODS
We searched electronic databases for articles on Alzheimer's disease, Klotho protein, cognitive function and aerobic exercise published between 2012 and 2022 from Google Scholar, PubMed, ProQuest, ScienceDirect, and SpringerLink. The articles have been chosen from trusted publisher. We checked the article from Scimagojr to find the reputation of the publisher and we chose the good reputation publisher. Articles not related to Alzheimer's disease, Klotho protein, cognitive function and aerobic exercise were excluded.
RESULTS
The articles from five databases have been searched thoroughly. We found 1.788 results from the initial search strategy. From these results, we screened 452 results by title, and then 16 results which eligible. Finally, we included 8 journals. We selected the journals based on the title and its relevancy to our topics, and to know the journal reputation index, we used https://www.scimagojr.com/.
Identification of studies via electronic databases
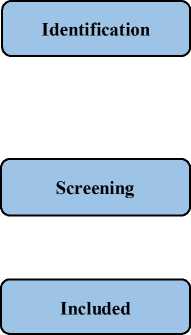
Results identified from (n = 1.788) Google Scholar (n = 1.650) PubMed NCBI (n = 3) Science direct (n = 40) ProQuest (n = 70)
Springer Link (n = 25)
Records screened by title (n = 452 )
Reports assessed for eligibility (n = 16 )
Studies included in review (n = 8 )
Figure 1. The diagram process of the study selection
Table 1. Data Extraction of Literature Review Results
|
No |
Author, Year of Research |
Participant/Research Groups |
Outcomes |
Intervention and Research Purpose |
Exercise Duration |
Frequency |
Research Methods |
Research Result | |
|
1. |
Gaitan et al., 2021 |
25 participants had a mean age of 64.9 years, had parental history of Alzheimer Disease, and all were cognitively healthy. |
BDNF, CTSB, and Klotho Quantification by Enzyme- Linked Immunosorbent Assay (ELISA). |
Intervention: Exercise training. Research purpose: To examine that circulating BDNF, CTSB, and klotho would increase following exercise training and correlate with cognition and metabolomic markers of brain health. |
26 weeks |
Less than minutes week |
150 per |
Randomized control trial |
The analyses indicate metabolic regulation of exercise-induced plasma BDNF changes and provide evidence that CTSB is a marker of cognitive changes in late middle-aged adults at risk for dementia. |
|
2. |
Mostafidi |
30 Athletes football |
Serum |
Intervention: |
It is not |
Exercise |
Randomized |
The plasma free Klotho | |
|
et al., 2016 |
players males (age range 18-22 years) and 28 healthy young males (age range 1827 years). |
concentrations of Klotho, cholesterol and triglycerides were measured with the ELISA technique. |
Reguler aerobic exercise Research purpose: To measure and compare the circulating Klotho levels in the serum of trained athletes and in healthy, non-athlete controls. |
known whether this elevation continues over time. |
training one day before testing. |
control trial |
concentration was significantly higher in the athlete group compared to the non- athletes and no significant differences between the groups for total cholesterol, triglycerides, and blood pressure. | ||
|
3. |
Morishima et al., 2021 |
12 untrained young male age 20 years. Nonsmokers, with no history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease, no taking |
Blood pressure and klotho concentration were measured with ELISA. |
Intervention: Resitance exercise Research purpose: To investigate the impact of acute resist- ance exercise on the |
One day when doing research. |
4 resistance exercise. |
sets |
Randomized control trial |
Klotho concentrations were significantly increased following a single bout of resistance exercise |
4.
5.
|
medications and supplements. |
Klotho response in serum. | ||||||
|
Iturriaga et |
91 Men aged 18–55 |
SαKl levels |
Intervention: |
One day |
Five sets of 20 |
Randomized |
Plasma levels of the klotho |
|
al., 2021 |
years, non-smokers, cardiorespiratory or strength training at least three times a week for 6 months or longer, and no musculoskeletal injuries sustained in the past 6 months. |
quantification by ELISA |
Cardiorespiratory exercise session and strength exercise session Research purpose: Compare the acute SαKl response to different exercise interventions, cardiorespiratory, and strength exercise in healthy, physically active men and to examine the behavior of SαKl 72 h after acute strength exercise. |
when doing research. |
jumps with 2 min rest intervals between sets and 10 s of rest between each jump and took approximately 1 hour to complete. |
control trial |
protein increased after acute cardiorespiratory exercise yet diminished immediately after an acute strength exercise session, only to increase with respect to pre-exercise levels 24 h later. |
|
Ji et al., |
30 rats Male 3-month- |
mRNA |
Intervention: |
48 weeks |
60 min/day, 5 |
Randomized |
Klotho mRNA and protein |
|
2018 |
old (weight, 320-350 g). |
expression levels of Klotho were measured using a thermal cycler. |
intermittent aerobic exercise (IAE) or continuous aerobic exercise (CAE) Research purpose: To determine whether aerobic exercise could influence the expres- sion of Klotho, decrease reactive oxygen species (ROS) and prolong life span. |
days/week |
control trial |
expression levels were increased significantly following aerobic exercise compared with controls. | |
|
6. |
Amaro et |
— 80 healthy adults |
S-Klotho plasma |
Intervention: |
12 weeks |
— Less than 50 |
Randomized |
The S-Klotho plasma levels |
|
al. 2018 |
(50% women) aged 45–65 years old. |
levels. |
aerobic training Research purpose: to determine the effect of different training modalities on the S-Klotho plasma levels in sedentary healthy adults. |
min/week. |
control trial |
could be related to physical exercise inasmuch physical exercise is involved in physiological pathways that regulate the S- Klotho plasma levels. | ||
|
7. |
Matsubara et al., 2014 |
69 healthy postmenopausal women (50-76 years old) |
Plasma Klotho concentration |
Intervention: aerobic training Research purpose: to examine the effects of aerobic training to plasma Klotho concentration and arterial stiffness |
12 weeks |
>3 days/ week (2-3 sessions were supervised, and additional home-based training). Initially 30 min/day (60% of the heart rate), and increased to 40-60 min/day (70-80% of the maximal heart rate) |
Cross sectional |
The aerobic exercise is increasing plasma Klotho concentrations and carotid artery compliance. Meanwhile, it is decreasing the β stiffness index |
|
8. |
Baghaiee et al., 2018 |
40 wistar rats, young (4 month old) and middle aged rats (13 15 month old) |
Plasma Klotho level |
Intervention: aerobic training Research purpose: to examine the the effect of aerobic training on serum levels of Klotho and cardiac tissue intracellular reactive oxygen species (ROS) |
8 weeks |
Using rodent's treadmill. Initially 11 m/min,slope 0%, distance travelled 180 meters; increased to 14 m/min during 13 minutes, slope 0%, distance |
Experimental |
The moderate aerobic exercise is increasing plasma Klotho, and decreasing oxidative stress, ERK1/2, P38, and fibrosis |
|
production, MAPK pathway, fibrosis and concomitant changes in middle aged rats |
travelled 460 meters during 34 min in fourth week; 16 m/min, slope 0%, distance travelled 830 meters during 54 min in eight weeks. |
DISCUSSION
-
a. Klotho and its function
Klotho (KL) protein is the anti-aging protein, encoded by KL genes. KL family members are α-KL, β-KL, and γ-KL genes. β-KL is found in adipose tissue, liver, kidney, pancreas, gut, and spleen. β-KL regulates energy metabolism and mediates the fibroblast growth factor (FGF) activity, FGF15 and FGF210. Meanwhile, γ-KL activates FGF19 and FGF23. This KL is found in kidney, skin, and brown adipose tissue21. The term KL in this literature refers to α-KL.
Klotho protein expression begins in utero, and increases from infancy to adulthood. The expression of KL decreases with age starting at 40 years of life22. KL has a biological role in decreasing oxidative stress, suppressing inflammation, and regulating phosphate, vitamin D, and energy metabolism23. In mice, overexpression of KL increases lifespan. Contrary to KL-deficient mice, it becomes prematurely aged, shorter lifespan, and dementia. The aging-related disorders are also apparent in the deficiency of KL, such as loss of muscle and fat mass, skin thinning, infertility, atherosclerosis, osteoporosis, and ectopic calcification24,25. KL has the capacity to decrease the expression of pro-inflammatory cytokines (IL-6 and IL-8) which relate to cells senescent; thus it has the anti-inflammatory and anti-aging effects10.
Klotho protein is found in the kidneys, brain, and pituitary glands. It is found in small amounts in the skeletal muscle, urinary bladder, ovaries, and testes. KL is the part of the FGF receptor (FGFR). Its ligands are bound to FGF 19, FGF 21, and FGF 23. It has a contribution in phosphate excretion in the kidneys10.
There are two types of KL, bound with membrane and secreted-forms. The latter is formed by chaining or sloughing of transmembrane KL with the help of membrane-anchored proteases, disintegrins and metalloproteinases domain-containing proteins, ADAM 10 and 1725,26. Transmembrane KL is found on the cell surface, generated by the kidney, and expressed in the brain, choroid plexus and neuron24.
The membrane bound KL is an obligate receptor for FGF23, known as the KL co-receptor in kidney26. Klotho protein and FGF23 form a complex called the FGF23/KL complex. It has metabolic functions in many tissues such as the kidney, heart, and brain. FGF23/KL complex increases synapses density in hippocampal neurons. It regulates mineral ions such as calcium and phosphate homeostasis21. Klotho protein increases neuronal plasticity factors and memory, and alleviates anxiety18.
Shed KL is found in blood, urine, and cerebrospinal fluid. ADAM 10 cleaved KL to 130 kDa, ADAM 17 cleaved KL to 68 kDa. The first cut cleaved KL to 130 kDa and contained KL1 and KL2 fragments. The second cut cleaved the KL to 68 kDa and localized it in KL1 and KL2 segment25. Insulin and low extracellular calcium stimulates shed KL24. Secreted KL has biologic effects in the body and is expressed by the kidney. It is a predominant human RNA transcript27.
-
b. Aerobic training increases Klotho protein level
Aerobic exercise is important for improving physical function and quality of life. Aerobic exercise has been shown to delay aging, impair cognitive function, and reduce neurological function. The KL gene is expressed in the brain and kidneys. When kidney or brain disease is present, KL protein expression is reduced. Decreased expression of the KL gene increases the aging rate of neurons and causes cell degeneration. Increased expression of KL protein helps clear ROS damage in the body28,29. Lack of KL gene expression is associated with aging phenotypes such as atherosclerosis, decreased bone mineral density, sarcopenia, skin atrophy, and cognitive impairment30. Physical activity and exercise can delay or prevent the development of Alzheimer's disease. KL protein is one of the systemic biomarkers involved in learning and memory19.
KL protein deficiency causes premature aging. Insulin / IGF-I is an important signaling pathway in aging research. KL protein indicates IGF-I and insulin resistance. S-KL inhibits IGF-I and insulin receptors by inhibiting tyrosine phosphorylation of both the receptor and its downstream signaling protein. Blockade of s-KL-induced IGF-I signaling increases resistance to oxidative stress and thus improves immunity. According to Saghiv the level of circulating s-KL is similar to that of a well-trained young athlete. The response depends on the level of aerobic exercise. Athletes who were trained in aerobic s-KL levels increased significantly and IGF-I levels decreased compared to those who were not active. Aerobic exercise is proven to increase s-KL, while anaerobic exercise does not affect the
expression of s-KL. Elderly individuals with aerobic exercise have a longer life expectancy than those who are not active20.
According to Ji, exercise prolongs aging and life by increasing KL protein expression in brain and kidney tissue. Any form of aerobic exercise can be selected based on individual condition28. With moderate-intensity aerobic exercise, it was effective to change pathological hypertrophy to physiological cardiac hypertrophy in rats. The effect of aerobic exercise can increase KL and reduce oxidative stress31. The effects of skeletal muscle on many endocrine organs have become a growing subject of research in recent years. The association between KL expression and skeletal muscle contraction may help explain the anti-aging effects of physical activity. Further investigation is needed to determine whether skeletal muscle contractile activity can cause serum secretion of KL into the bloodstream or the myokines that cause KL expression in the kidneys or brain. This can lead to the development of rehabilitation programs that are targeted and specifically designed to counter the effects of aging27.
In post menopausal women, the aerobic exercises can increase KL and vasodilate vascular. The aerobic exercises, such as cycling and walking, should be done in 30 minutes a day at relatively low intensity for 12 weeks. While in athletes, the football players, the aerobic exercise also increases KL protein level compared to non-athletes groups32,33. Moderate-intensity aerobic exercise for 8 weeks may increase KL serum. The increase in KL serum is in response to exercise to reduce oxidative stress. It also leads to a reduction in abnormal cardiac hypertrophy and the development of physiological hypertrophy29. According to Matsubara, aerobic exercise causes an increase in KL concentrations in plasma. His research showed that 12 weeks of moderate aerobic exercise was positively correlated with KL plasma levels. These results suggest a possible role for secret KL in modulating exercise-induced arterial stiffness32.
Klotho protein secretion can reduce apoptosis and cellular senescence that impairs endothelial function in the vascular endothelium. Aerobic exercise increases KL plasma concentration and reduces arterial stiffness. The serum KL concentration is increased significantly in response to a single resistance exercise as well as being proven in long-term aerobic exercise. This suggests that KL serum may be related to total muscle mass and muscle strength in the body32,34. The loss of klotho protein relates to muscle by reducing the proliferation and regeneration of muscle stem cells. Hence, it reduces the capacity of skeletal muscle to regenerate. The finding is similar with aging muscle, which has reduced regeneration capacity35. The acute increase in KL serum concentrations after resistance training may contribute to an increase in the baseline of KL serum after long-term exercise34. According to Iturriaga, levels of soluble KL increase in response to a single cardiorespiratory exercise but decrease immediately after strength training, levels increasing after 24 hours of exercise36.
-
c. Klotho improves Cognitive Function
Klotho protein has been linked to cognitive function in numerous studies. Improved learning and memory tasks seen in transgenic mice with systemic over expression of KL. LTP (Longterm Potentiation) is a type of synaptic plasticity GluN2B that is complemented by N-methyl-D-aspartic acid (NMDAR) receptors and is a major role in learning and memory processes in mice. The KL-mediated effects were demonstrated to be abolished when GluN2B was blocked. Klotho protein has an effect on young mice as well. According to the study the increased KL can improve cognition and avoid cognitive deficiencies at various periods of life17.
The polymorphism KL-V(F352V)S(C370S) is linked to the effect of KL on cognition. People with the KL-VS polymorphism (a mutation in the gene that codes for a protein that helps govern nerve growth) have greater cognitive function than people without the polymorphism, according to the Hillblom Aging Study. A study conducted by Zhou et al. is corroborated this finding. The KL polymorphism is made up of six SNPs, three in exons and three in introns, according to a study. Amino acid substitutions are thought to be caused by two frequent polymorphisms in the exon of the F352V and C370S genes. In human embryonic kidney cells, expression of this polymorphism increased development KL-FGF receptor complex and increased FGF23 signaling37. Serum KL levels were higher in heterozygous carriers KL-VS polymorphism than in non-carriers. Higher scores on measures of semantic competence, categorical competence, and modify assessments were linked to higher serum KL levels in and out of the workplace38.
The hippocampus controls of episodic memory storage and retrieval. KL deficiency damages the cholinergic nervous system in the hippocampus, according to Park et al., evidenced by a significant increase in AChE activity and AChE gene expression and also a significant decrease in M1-mAchR gene expression, M1nAchR binding, Ach levels, ChAT activity, and ChAT gene expression. KL insufficiency at cholinergic nerve terminals of presynaptic neurons expressing M1mAChR in the hippocampus causes these alterations in the cholinergic nervous system. KL insufficiency lowered the expression of PKCbII, p-ERK, p-CREB, and BDNF, as well as NMDAR-dependent LTP, indefinitely39. KL has a neuroprotective impact via the redox system, according to recent studies. Through activation of the thioredoxin/peroxiredoxin pathway, the Klotho protein protects hippocampus neurons from oxidative damage caused by glutamate and β-amyloid40.
Low levels of KL promote malfunction of the hippocampal circuits involved in consolidating LTM (long-term memory), but not of the prefrontal circuits involved in STM (short-term memory), according to KL-deficient mice, who performed worse 24 hours after exercise than one hour after exercise. Experiments with mice overexpressing (KL-OE) revealed that KL-OE boosted STM and LTM at an age-independent manner. Young and middle-aged KL-OE mice performed better at Morris water maze tests, hippocampal-dependent spatial memory tests, and context-based fear conditioning tests. The KL-OE mice performed better at the Y-maze test, which is a sort of STM that measures working memory17.
The NMDA receptor with higher expression of the GluN2B subunit improves memory in old mice. The NMDA receptor with increased expression of the GluN2B subunit improves synaptic memory and transmission in the frontal lobe, caudate nucleus, and hippocampus of the elderly mouse. It implies that it can be improved. When it is compared to old mice, the mice with enhanced GluN2B expression in all brain areas have higher long-term spatial memory, and the young mice have similar memory on different training days for different injection sites. In the hippocampus, increased expression GluN2B component improves NMDA receptor mediated synaptic transmission41.
The anti-aging protein KL may help oligodendrocytes in the central nervous system mature and myelinate. Cognitive impairments are caused by the lack of KL expression42. Increased levels of KL in the brain have been linked to increased cognitive function in both humans and animals, according to the study. The link between KL and cognitive functions is explained by two theories: 1) KL promotes memory formation and hippocampus function via neuroprotective and antioxidant processes; 2) KL enhances hippocampal and prefrontal memory by modifying LTP via activation of the GLUN2B NMDAR subunit38.
-
d. Klotho and Alzheimer Diseases
Klotho protein has been related to increased longevity and cognitive function in the elderly43. Klotho protein is found in high concentrations in the kidneys and brain, where it is associated to a number of key biological processes11. Growth factor-mediated signaling, calcium homeostasis, synapse function, autophagy, and cell survival are just a few of the crucial biological processes that Klotho protein is engaged in44. Klotho protein concentrations have been associated with a longer lifespan and a decrease in cellular aging indicators (e.g. lower epigenetic age, higher telomerase activity)45. In 2002, Arking et al. discovered that one allele of the KL (KL) gene, KL-VS, is associated with human aging46. KL-VS is a haplotype with two linkage disequilibrium (LD) missense variants: rs9536314 (p.F352V) and rs9527025 (p.C370S)47. Some researchers believe that having just one copy of the KL-VS gene is associated with improved mental abilities and reduced Alzheimer's disease pathology. However, this theory has been disputed by other researchers41,42. A functional haplotype is formed in humans by variants of rs9536314 (F352V) and rs9527025 (C370S) in the KL gene (KL, 13q13.1). KL-VShet, a KL-VS haplotype with one copy instead of two, has previously been related to greater KL levels in the blood17. KL-VShet affects around 20% to 25% of the population and has been linked to improved cognitive performance throughout adulthood, higher frontotemporal gray matter volume in functionally normal persons, and lower mortality in cognitively normal people. These data suggest that KL is critical for maintaining cognitive performance and brain function as people age29,41.
According to recent studies, having high KL levels may aid in lowering the risk of Alzheimer's disease48. KL-VShet was linked to a lower risk of AD dementia and cognitive decline in elderly people carrying the ApoE 4 allele, the strongest genetic risk factor for AD dementia, possibly due to elevated levels of primary AD pathology like cortical beta-amyloid (Aβ) aggregation, according to a recent meta-
analysis47. KL-VShet was associated with reduced levels of Aβ deposition biomarkers in ApoE 4 carriers, suggesting that KL-VShet may have a direct impact on primary AD pathology levels49. In APOE-4 carriers with a single allele of KL-VS, Alzheimer's disease risk was lowered by 1.3 times compared to APOE-4 carriers without KL-VS. The authors also verified previous findings that KL-VS heterozygotes with APOE-4 had decreased amyloid deposition47. Heterozygotes with the -VS haplotype were likely to have greater levels of KL and lower AD risk than homozygotes, showing that KL has a beneficial potential47. Despite KL tends to promote amyloid removal through autophagic mechanisms which engage with APOE, no definitive relationship involving KL and APOE action in removal has been established at this time42,11.
Nevertheless, it's unknown if KL is linked to higher amounts of fibrillary tangles carrying pathologic tau, the major driver of Alzheimer's disease progression. In the presence of A deposition, the initial main pathology in AD, neurofibrillary tangles extend out from medial temporal lobe to higher cortical locations. Atrophy of gray matter and cognitive impairment are associated to the progressive formation of neurofibrillary tangles in the presence of Aβ pathology, and it is more predictive of such changes than Aβ50. Since tau pathology is so essential in medical care, it's critical to discover if the KL-VShet variant reduces the production of neurofibrillary tangles and thus cognitive decline in Alzheimer's disease at a given amount of Aβ deposition48.
The processes behind the relationship between KL and tau disease remain unknown. Insulin regulation, growth factor effects, notably FGF2345, redox system activation, and calcium signaling have all been linked to KL40. The association between the KL protein and decreased neurofibrillary tau might be understood by its function in autophagy, a process thought to be involved in the removal of AD pathology45,46.
CONCLUSIONS
Klotho protein is a large protein with anti-aging and neuro-protective properties. KL decreases in aging and brain with AD. Aerobic exercise has been found to delay aging and improve cognitive and neurological function. Exercise helps to slowdown the aging process and prolong life by enhancing KL protein level in brain tissues. Increasing KL improves and prevents cognitive deficits in various stages of life. Two theories of KL impacts cognitive functions. First, KL protein improves memory formation and hippocampus function through neuroprotective and antioxidant mechanisms. Second, upregulating of GLUN2B and NMDAR subunits by KL protein modulates LTP can impacting the formation of memory. KL enhances hippocampal memory and prefrontal memory. KL protein improves Alzheimer’s disease, by lowering the formation of neurofibrillary tangles, hence improving the cognitive decline in AD. The mechanism of KL protein explained by its role in autophagy. However, the mechanisms that link KL to tau pathology are still unknown. Through this article, the aerobic exercise is expected to improve cognitive function in AD patient by increasing the level of KL protein. The research about this topic is still a few, thus it becomes the limitation of this article. Further research is needed to prove the theories.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
-
1. Anastasiou E, Lorentz KO, Stein GJ, Mitchell PD. Prehistoric schistosomiasis parasite found in the
Middle East. Lancet Infect Dis. 2014;14(7):553-554. doi:10.1016/S1473-3099(14)70794-7
-
2. Yasobant S. Comprehensive public health action for our aging world: the quintessence of public health
policy. J Int Med Res. 2018;46(2):555-556. doi:10.1177/0300060517718452
-
3. Rockwood K, Bergman H. FRAILTY: A report from the 3rd joint workshop of IAGG/WHO/SFGG,
Athens, January 2012. Can Geriatr J. 2012;15(2):31-36. doi:10.5770/cgj.15.35
-
4. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: A review. Eur
J Intern Med. 2016;31:3-10. doi:10.1016/j.ejim.2016.03.007
-
5. Zvěřová M. Alzheimer’s disease and blood-based biomarkers – Potential contexts of use. Neuropsychiatr
Dis Treat. 2018;14:1877-1882. doi:10.2147/NDT.S172285
-
6. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020;16(3):391-460.
doi:10.1002/alz.12068
-
7. Jentoft ME, Erickson LA. Alzheimer Disease. Mayo Clin Proc. 2016;91(8):e117-e118.
doi:10.1016/j.mayocp.2016.06.002
-
8. Cammisuli DM, Innocenti A, Fusi J, Franzoni F, Pruneti C. Aerobic exercise effects upon cognition in
Alzheimer’s Disease: A systematic review of randomized controlled trials. Arch Ital Biol. 2018;156(1-2):54-63. doi:10.12871/00039829201816
-
9. Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise
interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI database Syst Rev Implement reports. 2018;16(3):752-775.
doi:10.11124/JBISRIR-2017-003551
-
10. Amaro-Gahete FJ, de-la-O A, Jurado-Fasoli L, Ruiz JR, Castillo MJ, Gutiérrez Á. Role of Exercise on S-
Klotho Protein Regulation: A Systematic Review. Curr Aging Sci. 2018;11(2):100-107.
doi:10.2174/1874609811666180702101338
-
11. Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019;15(1):27-44.
doi:10.1038/s41581-018-0078-3
-
12. Mytych J, Sołek P, Będzińska A, et al. Klotho-mediated changes in the expression of Atg13 alter
formation of ULK1 complex and thus initiation of ER- and Golgi-stress response mediated autophagy. Apoptosis. 2020;25(1-2):57-72. doi:10.1007/s10495-019-01579-z
-
13. Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR. Klotho overexpression improves amyloid-β
clearance and cognition in the APP/PS1 mouse model of Alzheimer’s disease. Aging Cell. 2020;19(10):1-17. doi:10.1111/acel.13239
-
14. Zhu L, Stein LR, Kim D, et al. Klotho controls the brain–immune system interface in the choroid plexus.
Proc Natl Acad Sci U S A. 2018;115(48):E11388-E11396. doi:10.1073/pnas.1808609115
-
15. Li D, Jing D, Liu Z, Chen Y, Huang F, Behnisch T. Enhanced expression of secreted α-klotho in the
hippocampus alters nesting behavior and memory formation in mice. Front Cell Neurosci. 2019;13(April):1-16. doi:10.3389/fncel.2019.00133
-
16. Gonzalez‐rodriguez M, Villar‐conde S, Astillero‐lopez V, et al. Neurodegeneration and astrogliosis in the
human ca1 hippocampal subfield are related to hsp90ab1 and bag3 in alzheimer’s disease. Int J Mol Sci. 2022;23(1). doi:10.3390/ijms23010165
-
17. Dubal DB, Yokoyama JS, Zhu L, et al. Life Extension Factor Klotho Enhances Cognition. Cell Rep.
2014;7(4):1065-1076. doi:10.1016/j.celrep.2014.03.076
-
18. Shafie A, Rahimi AM, Ahmadi I, Nabavizadeh F, Ranjbaran M, Ashabi G. High-protein and low-calorie
diets improved the anti-aging Klotho protein in the rats’ brain: the toxic role of high-fat diet. Nutr Metab. 2020;17(1):1-12. doi:10.1186/s12986-020-00508-1
-
19. Gaitán JM, Moon HY, Stremlau M, et al. Effects of Aerobic Exercise Training on Systemic Biomarkers
and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front Endocrinol (Lausanne). 2021;12(May):1-18. doi:10.3389/fendo.2021.660181
-
20. Saghiv MS, Sira D Ben, Goldhammer E, Sagiv M. The effects of aerobic and anaerobic exercises on
circulating soluble-Klotho and IGF-I in young and elderly adults and in CAD patients. J Circ Biomarkers. 2017;6:1-8. doi:10.1177/1849454417733388
-
21. Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol.
2018;19(1):1-12. doi:10.1186/s12882-018-1094-z
-
22. Citterio L, Carpini SD, Lupoli S, et al. Klotho gene in human salt-sensitive hypertension. Clin J Am Soc
Nephrol. 2020;15(3):375-383. doi:10.2215/CJN.08620719
-
23. Cheng YW, Hung CC, Fang WH, Chen WL. Association between Soluble α-Klotho Protein and
Metabolic Syndrome in the Adult Population. Biomolecules. 2022;12(1):1-9. doi:10.3390/biom12010070
-
24. Vo HT, Laszczyk AM, King GD. Klotho, the Key to Healthy Brain Aging? Brain Plast. 2018;3(2):183-
194. doi:10.3233/bpl-170057
-
25. Olejnik A, Franczak A, Krzywonos-zawadzka A, Ka M, Bil-lula I. The Biological Role of Klotho Protein
in the Development of Cardiovascular Diseases. 2018;2018. doi:10.1155/2018/5171945
-
26. Avin KG, Coen PM, Huang W, et al. Skeletal muscle as a regulator of the longevity protein, Klotho. Front
Physiol. 2014;5 JUN(June):1-9. doi:10.3389/fphys.2014.00189
-
27. Avin KG, Coen PM, Huang W, et al. HYPOTHESIS AND THEORY ARTICLE Skeletal muscle as a
regulator of the longevity protein, Klotho. Published online 2014. doi:10.3389/fphys.2014.00189
-
28. Ji N, Luan J, Hu F, et al. Aerobic exercise-stimulated klotho upregulation extends life span by attenuating
the excess production of reactive oxygen species in the brain and kidney. Exp Ther Med. 2018;16(4):3511-3517. doi:10.3892/etm.2018.6597
-
29. Baghaiee B, Barzegari M, Sadeghi Zali MH, Hakimi M. Effect of Exercise Training and Aging on Klotho
Signaling in the Heart. J Clin Res Paramed Sci. 2019;8(2). doi:10.5812/jcrps.95724
-
30. Amaro-Gahete FJ, De-la-O A, Jurado-Fasoli L, et al. Exercise training as S-Klotho protein stimulator in
sedentary healthy adults: Rationale, design, and methodology. Contemp Clin Trials Commun. 2018;11(May):10-19. doi:10.1016/j.conctc.2018.05.013
-
31. Baghaiee B, Karimi P, Siahkouhian M, Pescatello LS. Moderate Aerobic Exercise Training Decreases
Middle-Aged Induced Pathologic Cardiac Hypertrophy by Improving Klotho Expression, MAPK Signaling Pathway, and Oxidative Stress Status in Wistar Rats. http://fiji.sc/Fiji
-
32. Matsubara T, Miyaki A, Akazawa N, et al. Aerobic exercise training increases plasma Klotho levels and
reduces arterial stiffness in postmenopausal women. Am J Physiol - Hear Circ Physiol. 2014;306(3):348-355. doi:10.1152/ajpheart.00429.2013
-
33. Mostafidi E, Moeen A, Nasri H, Hagjo AG, Ardalan M. Serum klotho levels in trained athletes.
Nephrourol Mon. 2016;8(1):18-20. doi:10.5812/numonthly.30245
-
34. Morishima T, Ochi E. Impact of a single bout of resistance exercise on serum Klotho in healthy young
men. Physiol Rep. 2021;9(21):1-8. doi:10.14814/phy2.15087
-
35. Ahrens HE, Huettemeister J, Schmidt M, Kaether C, von Maltzahn J. Klotho expression is a prerequisite
for proper muscle stem cell function and regeneration of skeletal muscle. Skelet Muscle. 2018;8(1):1-14. doi:10.1186/S13395-018-0166-X/FIGURES/6
-
36. Iturriaga T, Yvert T, Sanchez-Lorente IM, et al. Acute Impacts of Different Types of Exercise on
Circulating α-Klotho Protein Levels. Front Physiol. 2021;12. doi:10.3389/FPHYS.2021.716473/FULL
-
37. Zhou TBT, King GD, Chen C Di, Abraham CR. Biochemical and functional characterization of the
klotho-VS polymorphism implicated in aging and disease risk. J Biol Chem. 2013;288(51):36302-36311. doi:10.1074/jbc.M113.490052
-
38. Abraham CR, Mullen PC, Tucker-Zhou T, Chen CD, Zeldich E. Klotho Is a Neuroprotective and
Cognition-Enhancing Protein. Vol 101. 1st ed. Elsevier Inc.; 2016. doi:10.1016/bs.vh.2016.02.004
-
39. Park SJ, Shin EJ, Min SS, et al. Inactivation of JAK2/STAT3 signaling axis and downregulation of m1
mAChR cause cognitive impairment in klotho mutant mice, a genetic model of aging. Neuropsychopharmacology. 2013;38(8):1426-1437. doi:10.1038/npp.2013.39
-
40. Zeldich E, Chen C Di, Colvin TA, et al. The neuroprotective effect of Klotho is mediated via regulation
of members of the redox system. J Biol Chem. 2014;289(35):24700-24715.
doi:10.1074/jbc.M114.567321
-
41. Brim BL, Haskell R, Awedikian R, et al. Memory in aged mice is rescued by enhanced expression of the
GluN2B subunit of the NMDA receptor. Behav Brain Res. 2013;238(1):211-226.
doi:10.1016/j.bbr.2012.10.026
-
42. Chen C Di, Sloane JA, Li H, et al. The antiaging protein klotho enhances oligodendrocyte maturation and
myelination of the CNS. J Neurosci. 2013;33(5):1927-1939. doi:10.1523/JNEUROSCI.2080-12.2013
-
43. Zhu Z, Xia W, Cui Y, et al. Klotho gene polymorphisms are associated with healthy aging and longevity:
Evidence from a meta-analysis. Mech Ageing Dev. 2019;178(October 2018):33-40.
doi:10.1016/j.mad.2018.12.003
-
44. Dubal DB, Yokoyama JS. Longevity Gene KLOTHO and Alzheimer Disease - A Better Fate for
Individuals Who Carry APOE ϵ4. JAMA Neurol. 2020;77(7):798-800.
doi:10.1001/JAMANEUROL.2020.0112
-
45. Wolf EJ, Morrison FG, Sullivan DR, et al. The goddess who spins the thread of life: Klotho, psychiatric
stress, and accelerated aging. Brain Behav Immun. 2019;80:193-203. doi:10.1016/J.BBI.2019.03.007
-
46. Seto M, Weiner RL, Dumitrescu L, Hohman TJ. Protective genes and pathways in Alzheimer’s disease:
moving towards precision interventions. Mol Neurodegener. 2021;16(1). doi:10.1186/S13024-021-00452-5
-
47. Belloy ME, Napolioni V, Han SS, Le Guen Y, Greicius MD. Association of Klotho -VS Heterozygosity
with Risk of Alzheimer Disease in Individuals Who Carry APOE4. JAMA Neurol. 2020;77(7):849-862. doi:10.1001/jamaneurol.2020.0414
-
48. Zeng CY, Yang TT, Zhou HJ, et al. Lentiviral vector–mediated overexpression of Klotho in the brain
improves Alzheimer’s disease–like pathology and cognitive deficits in mice. Neurobiol Aging. 2019;78:18-28. doi:10.1016/J.NEUROBIOLAGING.2019.02.003
-
49. Erickson CM, Schultz SA, Oh JM, et al. KLOTHO heterozygosity attenuates APOE4-related amyloid
burden in preclinical AD. Neurology. 2019;92(16):E1878-E1889. doi:10.1212/WNL.0000000000007323
-
50. Guo T, Korman D, Baker SL, Landau SM, Jagust WJ. Longitudinal Cognitive and Biomarker
Measurements Support a Unidirectional Pathway in Alzheimer’s Disease Pathophysiology. Biol Psychiatry. 2021;89(8):786-794. doi:10.1016/J.BIOPSYCH.2020.06.029
123
Discussion and feedback