The EXERCISE INCREASES BRAIN-DERIVED NEUROTROPHIC FACTOR LEVEL ON PARKINSON’S DISEASE
on
Sport and Fitness Journal
Volume 10, No.2, May 2022: 77-84
E-ISSN: 2654-9182
EXERCISE INCREASES BRAIN-DERIVED NEUROTROPHIC FACTOR LEVEL ON PARKINSON’S DISEASE
Stefani Krisanti1*, Alice Angelina Surya Perdana1, Jeane Cynthia Wongso1, Clara Angelia1, Yuki Octavia Wijaya1, Nila Wahyuni2, I Putu Gede Adiatmika2
-
1 Magister Program of Biomedical Science Anti Aging Medicine Medical Faculty Universitas Udayana, 80234, Denpasar, Indonesia
-
2 Physiology Department Medical Faculty Universitas Udayana, 80234, Denpasar, Indonesia Email : stefanikrisanti@gmail.com
ABSTRACT
With the current development of medical science today, anti aging medicine has developed rapidly, including in neuroscience aspect. Indicator of healthy aging is to maintain a good quality of life, include maintaining optimal brain function. One of lifestyle factors that can improve health, prevent chronic diseases, and maintain cognitive function is exercise. Exercise has been shown to increase brain-derived neurotrophic factor (BDNF), which acts as a biomarker of neuroprotective. In patients with Parkinson's disease was found a decrease in BDNF serum levels. BDNF plays a major role as neuroprotection and neurorestoration, its levels can be increased through regular exercises with moderate-intensity. Thus, it can be considered as adjunctive therapy in Parkinson's disease. This literature review is to explain the correlation between exercise and BDNF level in Parkinson’s Disease.
Keywords: brain aging; neurodegenerative disease; aerobic exercise; neuroprotection
INTRODUCTION
Physical exercise with moderate-intensity, such as aerobics, have been shown to be safe and have a positive effect on patients with neurodegenerative diseases or neurological disorders such as Parkinson's disease 1. Exercise has a positive effect on cardiovascular system, respiratory system, also increases cerebral blood flow, changes in the structure of central nervous system, and release of neurotransmitters. Recent studies reveal the role of the neurotrophin, namely BDNF, which increases during exercise 2. BDNF is thought to play a role in improving neuroplasticity in Parkinson's disease 3.
BDNF is a protein belonging to the neurotrophin group that plays a role in the function of the central nervous system (CNS) and the peripheral nervous system. BDNF works by influencing cell differentiation, nerve cell growth and development, synaptogenesis, and synaptic plasticity 4,5,6. Research that has been done suggests that decreased levels of BDNF may be related to cause of neurodegenerative diseases such as Parkinson's disease7. A meta-analysis review stated that moderate-intensity aerobic exercise had a significant effect on increasing BDNF levels 2. Therefore, this literature review aims to find out the correlation between moderate-intensity aerobic exercise with BDNF levels in Parkinson's disease.
METHODS
-
a. Methodology
We conducted a systematic search of articles on electronic databases using PubMed, Google Scholar, ProQuest, and ScienceDirect with a combination categories of neurodegenerative disease (Parkinson's disease), neuroprotective biomarkers (BDNF, brain-derived neurotrophic factor, neurotrophin), and exercise (physcial activity, aerobic) and sort the range of the year from 2012 to 2022.
Sport and Fitness Journal
E-ISSN: 2654-9182 Volume 10, No.2, May 2022: 77-84
^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^^_———————————__————————————————__——_——__——_———————————__——_——————_————__——_——__—_
Then, we checked the articles obtained in the Scimago Journal Rank (SJR) to find out the reputation of the journal sources. Scopus index of journals used are Q1 and Q2.
-
b. Material and procedure
The articles used were reviewed and analyzed according to the following criteria: studies conducted in humans or animals, in populations with Parkinson's disease, measuring serum BDNF, exercise interventions, using experimental, observational, or meta-analyses studies. Articles were excluded based on the following criteria: no serum BDNF measurement, no neurological impairment, no exercise intervention, or duplication.
RESULTS
Identification

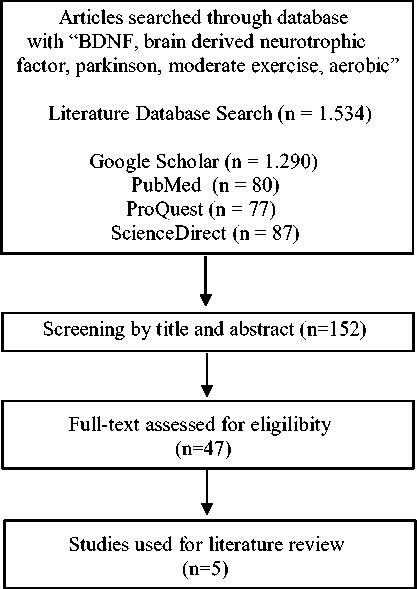
Figure 1. Literature review methodology
DISCUSSION
-
a. Parkinson’s Disease (PD)
PD is a degenerative neurological condition that can affect individuals as they age 8. It is known as the most common neurodegenerative disease after Alzheimer’s Disease 9,10. As human life expectancy increases, the number of people with PD rises, creating anguish for patients and caregivers, as well as a significant economic burden on society 8. Prevalence varies by age, gender, and geographic area, but the global estimate is 315 per 100.000 people 11.
A hallmark histological aspect of PD is the loss of nigrostriatal dopaminergic neurons in the substantia nigra 11,12, pars compacta 13. Neuroinflammation cause dopaminergic neurons degeneration 14. The symptoms of PD are divided into motoric and non-motoric symptoms 9. Resting tremor, bradykinesia (sluggishness and lack of mobility), and rigidity are hallmarks motoric symptoms of PD. Postural instability and gait abnormalities become more severe as the disease advances. While non-motoric symptoms that often occur are sleep disturbances, mental problems such as depression, and cognitive impairments include dementia 11,12.
PD is a degenerative disease that until now has no cure, there is only treatment to alleviate symptoms 11. Currently available pharmacological treatments such as dopamine replacement drugs can only treat motoric symptoms but cannot prevent or delay disease progression15. The effectiveness of pharmacotherapy declines as the disease progresses, thus necessitating the development of new therapeutic procedures16.
According to evidence, PD has a presymptomatic period of 10-20 years, or may be longer 15. This provides an opportunity to convert or slow the pathogenic progression from presymptomatic to PD. Identifying non-pharmacotherapies to postpone and diminish dopamine (DA) neuronal degradation and, as a result, the onset of PD symptoms is critical, in addition to the continuous research for pharmacological treatments. Non-pharmacotherapies, such as exercise, can be used in conjunction with current and future pharmacotherapy to improve symptoms, both motoric and non-motoric, also slow disease progression after the onset of PD symptoms. Exercise is particularly beneficial for improving cognitive function, reducing depression in people with PD 17 and also improving the side effects of anti-PD therapy such as wearing-off and dyskinesia 18. It has no adverse effects, non-invasive, and can be considered as non-pharmacological therapy for presymptomatic and clinical PD 19,20,21, 22.
-
b. Brain-Derived Neurotrophic Factor (BDNF)
The gene that regulates BDNF is located on chromosome 11. Synthesis of the pre-proBDNF precursor occurs in the endoplasmic reticulum, where it is transported to the Golgi apparatus and cleaved into the proBDNF isoform. This ProBDNF will be converted into mature BDNF (mBDNF) by endoproteases. The balance of proBDNF and mBDNF is influenced by the stage of brain development and brain region. During brain development, there are higher levels of proBDNF. While mBDNF functions as a neuroprotective and helps increase synaptic plasticity in adulthood 16. High expression levels and regulation of excitatory and inhibitory synaptic transmission are maintained by BDNF in the adult brain 23.
The term "neuroplasticity" refers to the ability of the CNS to change as a result of internal and external triggers. It is a procedure in which neurons adapt their function and structure in response to their environment 11. BDNF has an indirect neuroprotective effect on microglial activation by reducing nerve injury and inflammation 24.
The higher levels of BDNF in the brain which cross blood brain barrier, have been found in the hippocampus, amygdala, cerebellum and cerebral cortex, besides the lower levels of BDNF were found in organs such as heart, lung, and liver 23,25. The levels of BDNF in studies involving the same population, healthy control groups, and neurological populations vary at baseline. Age, sex, diurnal changes, food, metabolic and immune system problems influence BDNF levels 26. Furthermore, a frequent variant in the BDNF gene (Val66-Met) in humans may affect BDNF levels 27,28. BDNF Val66Met is a single nucleotide polymorphism that results in the substitution of valine for methionine 28. People with this polymorphism have a lower level of circulating BDNF because their activity-dependent release of BDNF is reduced. Participants in exercise intervention research are rarely genetically tested, however randomizing study participants may reduce this effect 2.
Increased BDNF levels are beneficial because they play a key role in neuroplasticity-related activities such as neurogenesis, dendritic growth, and long-term potentiation of neurons 26. BDNF supports
neuroprotection, increases dopaminergic neurotransmission, promotes dopaminergic neuron survival, and facilitates improved motor function in animal models of PD 16.
-
c. Neuroprotective Benefits of Exercise in People with Parkinson’s Disease
Over the past decade, there has been a growing amount of study emphasizing the potential of physical activity to enhance neuroprotection in PD 11,29,30. Animal studies using PD models have shown that physical activity increases plasticity processes and involved in neuroprotection mechanisms 31. Although animal studies have shown that exercise can cause neuroplastic changes, human studies are limited 11.
Aerobic exercise is a type of training that has been demonstrated to help people with neurological issues. After participating in the aerobic exercise program, those with stroke and PD experienced improvements in walking ability 32, functional ability, motor performance, and cardiorespiratory fitness 2. To explain why aerobic exercise is beneficial, several mechanism of processes have been proposed, including increased cerebral blood flow, altered neurotransmission, structural changes in the CNS, and altered arousal levels 33. Regular aerobic exercise is expected to increase BDNF expression across the CNS, which boosts neuroplasticity in the affected brain. According to a study and meta-analysis published in 2017, aerobic exercise was associated with higher levels of BDNF, as evaluated by peripheral blood, compared with standard treatment or no therapy in a study sample of stroke, multiple sclerosis, and PD 2. As part of neurological rehabilitation, regular aerobic exercise may help to raise BDNF levels, perhaps resulting in enhanced neuroplasticity and motor performance 34.
In animal models of PD, increased BDNF in response to aerobic exercise has been linked to improvements in symmetrical forelimb movement and balance 2. In human neurological populations, similar correlations between increased BDNF levels and improved motor performance have yet to be discovered. The impact on BDNF levels varies depending on the intensity or dose of exercise 2. Moderateintensity exercise consisted of session of 60 minutes length, three sessions per week performed for 8 weeks succeeded in increasing BDNF in the basal serum of patients with PD 35,36. Moderate-intensity exercise also improves functional capacity, gait, balance, and strength in patient 37,38,39.
A study showed that aerobic exercise performed 2-3 times per week had no effect on BDNF levels, whereas if performed 4-7 times per week had a significant effect on increasing BDNF. In this study, it was also stated that the average exercise time of 12.9 ± 3.9 hours did not give a difference in results in BDNF levels, while the average 20 hours of aerobic activity showed a significant increase in BDNF 2. In other research conducted by Lippi G in 2020, sufficient physical exercise, moderate-intensity aerobic exercise with a frequency of 2-3 sessions per week, a minimum duration of 30 minutes for 3 months can increase the release of BDNF which can maintain cognitive function 34. Other studies showed the benefits of treadmill exercise with frequency 5 times a week for 3 weeks will cause an increase in the expression of the BDNF gene in the brain that can improve PD by inhibiting the inflammatory pathway 40. The increase in BDNF shown after the exercise program in the neurological group was due to the cumulative dose of regular physical exercise 2.
Another study was conducted with a high-intensity interval training program using tandem cycling for 8 weeks, the results showed a significant increase in serum BDNF levels in PD, thereby increasing motor function, rigidity, bradykinesia, and inducing neuroplasticity 41 .
We also found the correlation between BDNF and depression in PD. Study by Szuhany et al. found that regular physical activity produced a two-fold effect on BDNF levels in patients with psychiatric illnesses such as depression than in healthy people. The results showed that the mean effect size of changes in BDNF in psychiatric patients is 0.40 versus 0.17 for healthy people 26. Exercise has been shown to have anti-depressant effects and also improves motor dysfunction and cognitive deficits in patients with PD 42,43.
Table 1. Description of study interventions and results
|
Author |
Population |
Design |
Intervention Group Activity |
Postprogram Outcome |
|
Angelucci et al.(44) |
Parkinson’s disease (n = 9) |
Prepost |
PD exercise 5 days/wk (3 sessions per day) 4 weeks 40 min ≤60% HRR |
BDNF NSD Pre-post p < 0.14 |
|
Frazzitta et al. (39) |
Parkinson’s disease (n = 24) |
RCT |
PD exercise vs PD no exercise 3 × 60 min 5 days/wk 4 weeks ≤60% HRR |
BDNF ↑ post ex 12.6% p = 0.017, NSD control p > 0.05 |
|
Marusiak et al. (35) |
Parkinson’s disease (n = 11), healthy controls (n = 11) |
QE |
PD exercise vs healthy no exercise 3 days/wk 8 weeks 40 min 68% HR max |
BDNF ↑ post ex 34% p = 0.035 (p < 0.05), NSD in healthy control p = 0.81 |
|
Fontanesi et al.(45) |
Parkinson’s disease (n = 16) |
Prepost |
15 times/wk 4 wks 60 min |
BDNF-TrkB signaling ↑ post ex p < 0.001 in the peripheral lymphocytes at the levels of receptors, intracellular mediators, and downstream effectors. |
|
Zoladz et al. (36) |
Parkinson’s disease (n = 12) |
Prepost |
3 times/wk 8 wks 60 min 60-75% HR max |
BDNF ↑ post ex 34% p = 0.03 |
PD: Parkinson’s disease; RCT: randomised controlled trial; QE: quasiexperimental; N: sample size; BDNF: brain-derived neutrophic factor; TrkB: Tyrosine receptor kinase B; HRR: heart rate reserve; HR: heart rate; NSD: nonsignificant difference
All these results show that variations in exercise dose can affect BDNF levels outcome. Previous studies have shown that adequate exercise will not only prevent PD in susceptible people, but also slow the progression of the disease, which will have a positive effect on the cognitive and psychomotor functions of PD patients 8. BDNF has been demonstrated to protect and restore dopaminergic neurons, making it a potential treatment for PD 16,46. Exercise-stimulated synthesis of endogenous neurotrophic factors may help patients with PD by protecting and restoring dopaminergic neurons or repairing the damaged cortico-basal ganglia motor control circuit 8. Physical exercise that is well-chosen can enhance BDNF levels in the blood and brain, thus can protect neurons from neurotoxic assaults to some extent, as shown in animal models 16,47. Exercise is a widely available, simple, non-invasive, side-effect-free, and cost-free therapy which should be recommended for vulnerable people as a preventative strategy and those with PD as a therapeutic element 8.
However, further researches are needed to determine the type, training variables (intensity, duration, frequency), as well as the underlying mechanisms that have a positive effect on improving clinical symptoms of PD.
CONCLUSION
Our literature review showed evidence that moderate-intensity physical exercise such as aerobics can increase BDNF levels in Parkinson's disease as measured by peripheral blood. BDNF exerts neuroprotective and neurorestorative effects on dopaminergic neurons. Proper and regular physical exercise can be considered as a component of rehabilitation therapy to help increase BDNF levels in people with Parkinson's disease, which induces increased neuroplasticity and facilitates improvement in motor function, which will ultimately help improve the patient's quality of life. Future studies can focus more on how physical exercise can induce BDNF expression, the cellular and molecular mechanisms underlying neurorestoration, and the factors that influence BDNF outcomes.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
-
1. Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, et al. Phase I/II randomized trial of
aerobic exercise in Parkinson disease in a community setting. Neurology. 2014;83(5):413–25.
-
2. Mackay CP, Kuys SS, Brauer SG. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in
People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017;2017.
-
3. Monteiro-Junior RS, Cevada T, Oliveira BRR, Lattari E, Portugal EMM, Carvalho A, et al. We need to move
more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses [Internet]. 2015;85(5):537–41. Available from: http://dx.doi.org/10.1016/j.mehy.2015.07.011
-
4. Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical Implications. Arch Med Sci.
2015;11(6):1164–78.
-
5. Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a
promising therapeutic agent in parkinson’s disease. Int J Mol Sci. 2020;21(3).
-
6. Hartmann D, Drummond J, Handberg E, Ewell S, Pozzo-Miller L. Multiple approaches to investigate the
transport and activity-dependent release of BDNF and their application in neurogenetic disorders. Neural Plast. 2012;2012.
-
7. Azevedo LV dos S, Pereira JR, Silva Santos RM, Rocha NP, Teixeira AL, Christo PP, et al. Acute exercise
increases BDNF serum levels in patients with Parkinson’s disease regardless of depression or fatigue. Eur J Sport Sci [Internet]. 2021;0(0):1–8. Available from: https://doi.org/10.1080/17461391.2021.1922505
-
8. Hou L, Chen W, Liu X, Qiao D, Zhou FM. Exercise-induced neuroprotection of the nigrostriatal dopamine
system in Parkinson’s disease. Front Aging Neurosci. 2017;9(NOV).
-
9. Callaghan AO, Harvey M, Houghton D, Gray WK, Weston KL, Oates LL, et al. Comparing the influence of
exercise intensity on brain - derived neurotrophic factor serum levels in people with Parkinson ’ s disease : a pilot study. Aging Clin Exp Res [Internet]. 2019;(0123456789).
-
10. Storstein OTA. Epidemiology of Parkinson ’ s disease Diagnosis of PD. J Neural Transm. 2017;(1).
-
11. Johansson H, Hagströmer M, Grooten WJA, Franzén E. Exercise-Induced Neuroplasticity in Parkinson’s
Disease: A Metasynthesis of the Literature. Neural Plast. 2020;2020.
-
12. Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity
of the nigrostriatal system in Parkinson’s disease. Brain. 2013;136(8):2419–31.
-
13. Choi HY, Cho KH, Jin C, Lee JE, Kim TH, Jung WS, et al. Exercise Therapies for Parkinson’s Disease: A
Systematic Review and Meta-Analysis. Parkinsons Dis. 2020;2020.
-
14. Małczyńska-Sims P, Chalimoniuk M, Sułek A. The Effect of Endurance Training on Brain-Derived
Neurotrophic Factor and Inflammatory Markers in Healthy People and Parkinson’s Disease. A Narrative Review. Front Physiol. 2020;11.
-
15. Noyce AJ, Lees AJ, Schrag AE. The prediagnostic phase of Parkinson’s disease. J Neurol Neurosurg
Psychiatry. 2016;87(8):871–8.
-
16. Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a
promising therapeutic agent in parkinson’s disease. Int J Mol Sci. 2020;21(3).
-
17. Hsueh SC, Chen KY, Lai JH, Wu CC, Yu YW, Luo Y, et al. Voluntary physical exercise improves subsequent
motor and cognitive impairments in a rat model of parkinson’s disease. Int J Mol Sci. 2018;
-
18. Xu X, Fu Z, Le W. Exercise and Parkinson’s disease. 1st ed. Vol. 147, International Review of Neurobiology.
Elsevier Inc.; 2019. 45-74 p.
-
19. Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC, Viña J. Exercise acts as a drug; the
-
pharmacological benefits of exercise Keywords health; dosing of exercise; contraindications of exercise; sport; training. 2012;
-
20. Burley C V., Bailey DM, Marley CJ, Lucas SJE. Brain train to combat brain drain; focus on exercise strategies
that optimize neuroprotection. Exp Physiol. 2016;101(9):1178–84.
-
21. Jackson PA, Pialoux V, Corbett D, Drogos L, Erickson KI, Eskes GA, et al. The Journal of Physiology
Neuroscience Promoting brain health through exercise and diet in older adults: a physiological perspective. J Physiol. 2016;594:4485–98.
-
22. Lauzé M, Daneault JF, Duval C. The Effects of Physical Activity in Parkinson’s Disease: A Review. J
Parkinsons Dis. 2016;6(4):685–98.
-
23. Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-Derived Neurotrophic Factor : A Key Molecule for
Memory in the Healthy and the Pathological Brain. 2019;13(August):1–25.
-
24. Szymura J, Kubica J, Wiecek M, Pera J. The immunomodulary effects of systematic exercise in older adults
and people with Parkinson’s disease. J Clin Med. 2020;
-
25. Marquez CMS, Vanaudenaerde B, Troosters T, Wenderoth N. High-intensity interval training evokes larger
serum BDNF levels compared with intense continuous exercise. J Appl Physiol. 2015;119(12):1363–73.
-
26. Kristin L. Szuhanya, Matteo Bugattia MWO. A meta-analytic review of the effects of exercise on brainderived
neurotrophic factor. J Psychiatr Res. 2015;60:56–64.
-
27. Mang CS, Campbell KL, Ross CJD, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke:
Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013 Dec;93(12):1707–16.
-
28. Cagni FC, Campêlo CL das C, Coimbra DG, Barbosa MR, Oliveira Júnior LG, Neto ABS, et al. Association
of BDNF Val66MET polymorphism with Parkinson’s disease and depression and anxiety symptoms. J Neuropsychiatry Clin Neurosci. 2017;29(2):142–7.
-
29. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity
targeting motor and cognitive circuitry in Parkinson’s disease. Vol. 12, The Lancet Neurology. 2013. p. 716– 26.
-
30. Hirsch MA, van Wegen EEH, Newman MA, Heyn PC. Exercise-induced increase in brain-derived
neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Vol. 7, Translational Neurodegeneration. 2018.
-
31. Real CC, Ferreira AFB. BDNF Receptor Blockade Hinders the Beneficial Effects of Exercise in a Rat Model
of Parkinson's Diesease. 2013;237:118–29.
-
32. Bang DH, Son YL. Effect of intensive aerobic exercise on respiratory capacity and walking ability with
chronic stroke patients: A randomized controlled pilot trial. J Phys Ther Sci. 2016;28(8):2381–4.
-
33. Gligoroska J, Manchevska S. The Effect of Physical Activity on Cognition - Physiological Mechanisms. Mater
Socio Medica. 2012;24(3):198.
-
34. Lippi G, Mattiuzzi C, Sanchis-Gomar F. Updated overview on interplay between physical exercise,
neurotrophins, and cognitive function in humans. Journal of Sport and Health Science. 2020.
-
35. Marusiak J, Zeligowska E, Mencel J, Kisiel-Sajewicz K, Majerczak J, Zoladz JA, et al. Interval training-
induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor: A repeated-measures, case series pilot study. J Rehabil Med. 2015;47:372–5
-
36. Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A, et al. Moderate-intensity interval
training increases serum brain-derived neurotrophic factor level and decreases inflammation in parkinson’s disease patients. J Physiol Pharmacol. 2014;65:441–8
-
37. Carvalho AO De, Souza A, Filho S, Murillo-rodriguez E, Rocha B, Carta MG, et al. Clinical Practice &
Epidemiology in Physical Exercise For Parkinson ’ s Disease : Clinical And Experimental Evidence. 2018;89-98.
-
38. Shu H, Yang T, Yu S, Huang H, Jiang L, Gu J, et al. Aerobic Exercise for Parkinson ’ s Disease : A Systematic
Review and Meta-Analysis of Randomized Controlled Trials. 2014;9(7).
-
39. Frazzitta G, Maestri R, Ghilardi MF, Riboldazzi G, Perini M, Bertotti G, et al. Intensive rehabilitation
increases BDNF serum levels in parkinsonian patients: A randomized study. Neurorehabil Neural Repair.
2014;28(2):163–8.
-
40. Nazif NN, Khosravi M, Ahmadi R, Bananej M, Majd A. Effect of treadmill exercise on catalepsy and the
expression of the BDNF gene in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson in male NMRI mice. Iran J Basic Med Sci. 2020;
-
41. Segura C, Eraso M, Bonilla J, Mendivil CO, Santiago G, Useche N, et al. Effect of a High-Intensity Tandem
Bicycle Exercise Program on Clinical Severity, Functional Magnetic Resonance Imaging, and Plasma Biomarkers in Parkinson’s Disease. Front Neurol. 2020;
-
42. Fan B, Jabeen R, Bo B, Guo C, Han M, Zhang H, et al. What and How Can Physical Activity Prevention
Function on Parkinson’s Disease? Oxidative Medicine and Cellular Longevity. 2020.
-
43. Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Haidar EA, Stringer T, et al. Exercise promotes the
expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5(JUN2016):1–21.
-
44. Angelucci F, Piermaria J, Gelfo F, Shofany J, Tramontano M, Fiore M, et al. The effects of motor
rehabilitation training on clinical symptoms and serum BDNF levels in Parkinson’s disease subjects. Can J Physiol Pharmacol. 2016 Apr;94(4):455–61.
-
45. Fontanesi C, Kvint S, Frazzitta G, Bera R, Ferrazzoli D, Di Rocco A, et al. Intensive Rehabilitation Enhances
Lymphocyte BDNF-TrkB Signaling in Patients With Parkinson’s Disease. Neurorehabil Neural Repair. 2016 Jun;30(5):411–8.
-
46. Da Costa RO, Gadelha-Filho CVJ, Da Costa AEM, Feitosa ML, De Araújo DP, De Lucena JD, et al. The
Treadmill Exercise Protects against Dopaminergic Neuron Loss and Brain Oxidative Stress in Parkinsonian Rats. Oxid Med Cell Longev. 2017;
-
47. Schaeffer E, Roeben B, Granert O, Hanert A, Liepelt-Scarfone I, Leks E, et al. Effects of exergaming on
hippocampal volume and brain-derived neurotrophic factor levels in Parkinson’s disease. Eur J Neurol. 2022;
84
Discussion and feedback