ANTI-INFLAMMATORY ACTIVITY OF PEGAGAN LEAVES (Centella asiatica (L.) Urban) EXTRACT CREAM
on

Journal of Pharmaceutical Science and Application Volume 4, Issue 1, Page 12-18, June 2022 E-ISSN: 2301-7708
ANTI-INFLAMMATORY ACTIVITY OF PEGAGAN LEAVES (Centella asiatica (L.) Urban) EXTRACT CREAM
Siti Mardiyah1*
1Faculty of Pharmacy, University of 17 Agustus 1945, Jakarta
Corresponding author email: anandatiwi10@gmail.com
ABSTRACT
Background: Pegagan leaves (Centella asiatica (L.) Urban) is an herbal plant family of Apiaceae containing the active ingredients of the triterpenoid group glycosides (asiaticoside, Asiatic acid, madekasat acid, madikassosida) centelloside, flavonoids and Asiatic acids. Inflammation is a complex reaction in tissues that involves a response to blood vessels and leukocytes. Objective: The purpose of this study was to find out the anti-inflammatory activity that occurs in the legs of mice in the form of preparation of pegagan extract cream (Centella asiatica (L.) Urban). Methods: Pegagan extract is done by the maceration method that uses ethanol 70%. Test animals used to squeak white males as much as 10 heads with a body weight of 20-30 grams. Test animals were divided into 5 treatment groups, namely the pegagan extract group with a dose of 2 g/kg BB; 4 g/kg BB; 6 g/kg BB, positive control (Betason cream 0.1%), and negative control (pegagan extract 2 g/kg BB). 1 hour before treatment of squeaky soles is inducted with a 1% carrageenan solution subplantarically. Results: Measurement of edema volume on the soles of the feet of mice is observed every 1 hour for 6 hours. The results showed that pegagan extract cream with a dose of 6 g / kg BB showed the maximum percentage of inhibition edema that is at the 6 hour with a result of 80,2%. The results of the data analysis of the percentage of inhibition of Centella asiatica extract from the treatment group Negative Control-Formula II and Negative Control-Formula III showed significant differences (p<0.05). Conclusion: Pegagan leaves extract has an antiinflammatory effect on feet of mice.
Keywords: Anti-inflammatory, Pegagan leaves, Centella asiatica (L.) Urban
INTRODUCTION
Centella asiatica (L.) Urban is one of the medicinal plants that has been widely used in the community. Pegagan leaves contain active ingredients such as triterpenoid glycosides (especially asiaticosides, asiatic acid, madecasate acid, and madikassosida)[1]. The main contents of pegagan leaves are asiaticoside, centelloside, madecassoside, flavonoids and asiatic acids[2]. Asciaticosides are mostly dissolved by 70% ethanol so this solvent is often used in the manufacture of extracts[3]. Empirically, pegagan leaves are used to treat inflammation, and wounds, increase immunity, lower blood pressure, increase appetite, and improve memory. According to research, pegagan leaves
herbs at a concentration of 1.5% can accelerate wound healing more effectively[4].
Inflammation is a complex reaction in tissues involving responses to blood vessels and leukocytes. An antiinflammatory mechanism for destroying necrosis tissue that has the intrinsic ability to damage normal tissue. When inflammation is not on target and damaged tissue, it can cause damage and disease[5]. Anti-inflammatory is a designation for agents/drugs that work against or suppress the inflammatory process. Nonsteroidal anti-inflammatory inhibits cyclooxygenase which converts arachidonic acid into PGG2 and PGH2[6].
METHODS
1. Instruments
The instruments used in this study are rotary vacuum evaporator, maceration vessel, blender, wood stirrer, stirring rod, porcelain cup, mortar and stamper, water handler, digital scales, beaker glass, measuring glass, Sudip, spatula, napkin, flannel and container (cream pot), objectglass, watch glass, measuring glass, mixer rod, digital pH meter, scale boy, Brookfield viscometer, glass object, Tringle Brand, injection spuite (Terumo), needle (Terumo), stopwatch (Casio), mice scales (Kern), glassware (Pyrex), observation cage, healthy white squeak (Mus musculus) with a standard weight of 20-30 grams, aged 2-3 months who have a healthy and active physical condition.
The ingredients used for the study are pegagan leaves leaf Simplicia, 70% ethanol, Cera alba, paraffin liquid, glycerin, TEA, aquadest, methyl paraben, propyl paraben, 1 gram caragenan, NaCl 0.9%. Betason cream comparison ingredient 0.1%.
Plant Determination
Determination of plants aims to find out the authenticity of the material to be tested. Plants used for determination are whole plants. Observations are made on the roots, stems, leaves, and flowers and then matched with the determination key. Determination of the Pegagan leaves plant is carried out at the Center for Biological Research (LIPI) located at km.46, Jalan. Raya Bogor, Cibinong, Bogor, West Java. Working procedures
The research conducted is an experimental method in the laboratory with the following stages:
-
a. Plant determination
Determination of plants aims to find out the authenticity of the material to be tested. Plants used for determination are whole plants. Observations are made on the roots, stems, leaves, and flowers and
then matched with the determination key. Determination of the Pegagan leaves plant is carried out at the Center for Biological Research (LIPI) located at km.46, Jalan. Raya Bogor, Cibinong, Bogor, West Java.
-
b. Manufacture of pegagan leaves
Simplicia
Fresh pegagan leaves are then sorted from the impurities that are still attached, then pegagan leaves were washed thoroughly under running water. Then drying was done by aerated to dry. The drying lasts for five to six days. Simplicia that has been dried was drunk with a grinding machine (blender) and sieved with no 20.
-
c. Extraction
500 grams of pegagan leaves leaf Simplicia powder(Centella Asiatica (L.) Urban) is soaked using 70% ethanol 1.5 liters for 3 days accompanied by regular stirring. Leaf maceration was concentrated with vacuum drying and rotary evaporator at a temperature of 40 ° C until obtained a thick extract.
-
d. Phytochemical screening
Alkaloid test
The alkaloid test consists of 3 reagents, namely Mayer reagent, Magner reagent and Dragendorff reagent. A sample of 1 ml is dripped 1-2 drops of Mayer reagent, if positive white deposits occur. 12 drops of Wagner reagent, if positive brown deposits and Dragendorff reagent, if positive red deposits occur.
Flavonoids test
Into 5 ml extract add powder Mg, 2 ml alcohol solution-HCl (1:1) and 5 ml solution of amyl alcohol. Shake and observe the discoloration. If there is a red, yellow or orange color on the amyl layer of alcohol then positively there are flavonoids.
Terpenoid test
Into 1-2 ml of extract on the test tube is added 2 drops of anhydride acetate and stirred. Then drop 1-2 drops of concentrated H2SO4 and observe the color formed. Note the color that was formed at
the time of dripping and the color changed after a while. This test is positive when there is a change in color red to green to purple to blue.
Tannin test
A number of extracts on porcelain plates added a few drops of 1% FeCl3 solution. The tannin group is positive if there is a color change to purple or black. Saponin test
A total of 10 ml of the extract is inserted into a vertically shaken test tube for 10 seconds, if a 1-10 cm foam is formed stable for 10 minutes then the saponins are positive. Next, add 1 drop of HCL 2N solution, saponins are positive if the foam does not disappear.
-
e. Rendemen examination
The randemen value was obtained by dividing the weight of the extraction result by the initial weight of Simplicia. Calculation of randemen that can be known the value of eccentricity of each gram of extract thick with Simplicia (Dirgen BPOM, 2000).
-
f. Manufacture of cream base with several concentrations, namely 2 g / kg BB; 4 g / kg BB; and 6 g / kg BB.
The cream base used is type A / M (oil phase), the use of the cream base is because it is not easy to wash and does not dry easily because of slow evaporation of water so that there is the formation of a thin layer of protective oil that remains on the surface of the skin so that the active substance in the cream base can be absorbed properly. The cream base will be made with 3 concentrations of pegagan leaves leaf extract, namely a
concentration of 2 g / kg BB,, 4 g / kg BB,and 6 g / kgBB.. Basically, it has 3 different concentrations that are to find out the concentration of how much of an inflammatory power effect occurs on the edema of the squeaking feet. The cream formula design of pegagan leaves leaf extract can be seen in table 1.
|
Table 1 Pegagan leaves formulation design |
extract cream | ||||
|
Material |
Formula (gram) |
Control (-) |
Control (+) | ||
|
FI |
F II |
F III | |||
|
2 |
4 |
6 | |||
|
Pegagan extract |
g/kg |
g/kg |
g/kg | ||
|
BB |
BB |
BB | |||
|
Cera |
10 |
10 |
10 | ||
|
Alba | |||||
|
Paraffin |
10 |
10 |
10 | ||
|
Liquid |
Pegagan |
Betason | |||
|
Glycerin |
5 |
5 |
5 |
Extract |
Cream |
|
TEA |
1 |
1 |
1 |
2 g/kg |
0,1% |
|
Methyl paraben |
0,12 |
0,12 |
0,12 |
BB | |
|
Propyl Paraben |
0,05 |
0,05 |
0,05 | ||
|
Aquadest ad |
50 |
50 |
50 | ||
Cream making starts by weighing all the necessary ingredients according to the calculation. Put Cera alba and paraffin liquid into a porcelain cup, then melt over the water handler. Prepare the lumpang and heat sorting. Heating on lumpangs and sorting to speed up the mixing of materials evenly. Dissolve nipagin with hot water using Erlenmeyer. The fused tea, glycerin, and nipasol are mixed into a nipagin solution, stirring until homogeneous and becoming a phase of water. Put the water phase into the hot lumpang. After melting, the melting results are inserted little by little into lumpangs that have contained water phases, grinding until homogeneous and cold so that a cream base is formed. Add the pegagan leaves leaf extract little by little while being gnawed until homogeneous and becomes a half-solid mass (cream). Remove the cream from the lumpang and then put it in a container (plastic pot).
-
h. Physical evaluation of cream preparations
Organoleptic test
According to the Ministry of Health, the specification of the cream that must be met is to choose a half-solid shape, the color must be in accordance with the specifications at the time of the initial manufacture of the cream and the smell was not rancid[7-11].
Homogeneity Test
The test of the homogeneity of the preparation is done by means of the cream applied to a piece of glass or other suitable transparent material must show a homogeneous arrangement. Homogeneous creams are characterized by the absence of lumps in the polishing results, a flat structure and has a uniform color from the starting point of polishing to the end point of polishing. The cream tested was taken three places, namely the top, middle and bottom of the cream container[7-11].
Viscosity Test
The preparation is put in a glass. The examination is carried out using a Brookfield viscometer using the appropriate spindle, then inserted into the preparation until the limit mark was on the spindle s64[7-11].
pH test
Measurement of pH values using a pH meter aid dipped in 0.5 g of cream. A good cream pH value is 4.5-6.5 or in accordance with the pH value of human skin[7-11].
Scatter Power Test
Scatter power testing were done by placing 0.5 g of cream between two transparent object plates that were given a load of 100 g. Measurement of the spread power diameter was done after the cream does not re-spread or approximately 1 minute after the load. A good cream scatter power diameter is between 5-7 cm[7-11].
Prepared test animals that will be used were mice (Mus musculus). Selected healthy mice and have a standard body weight of 20-30 grams. It is then divided
into 5 groups, each consisting of 2 male mice[7-11].
Preparation of karagenine solution 1% A total of 0.1 grams of karagenin is weighed, and dissolved in a physiological solution of NaCl up to 10 ml (NaCl physiological solution 0.9%). Cream compared to Betason cream 0.1%[7-11].
Inflamation induction
Inflammation is made by injecting as much as 0.1 ml of 1% karagenine solution subplantar on the soles of the squeaky feet. The soles of the squeaking feet are marked as limited to the ankles and measured the volume of edema with a plestismometer[7-11] .
The data obtained will be analyzed with the Test of Homogeneity of Variance Levene test to find out whether the population of the data tested has homogeneous variants or not. If the data is distributed normally and homogeneously, it will continue the One-way ANOVA test and continue the Tukey HSD test to find out the differences obtained, data analysis is done with the SPSS program.
RESULTS
After anti-inflammatory testing with the manufacture of edema on the feet of mice by swinging it subplantarly with a carrageenan solution that was previously dissolved first with NaCl 0.9%. By injecting carrageenan can provide the volume of edema on the soles of the feet of the squeak, it is in accordance with the theory that the fastest carrageenan causes swelling with a gel shape and not hard[12]. The use of caragenan in this study can provide the effect of swelling and prostaglandin synthesis 4-5 times and the volume of edema formed on the soles of the squeaking feet is more visible. Then take a measurement of the volume of edema on the soles of the squeaky feet using a pletismometer to assess the effects
of inflammation. Measurements of the volume of the squeaking feet are calculated for 6 hours after induction using a caragenan in each group consisting of 2 males. After obtaining the results of volume measurement data in each group, then determined the average volume of edema listed in table 2.
Table 2 shows a decrease in foot volume after extract preparations of various concentrations, positive control and negative control characterized by a decrease in the volume of inflammation during examination using a plestimometer for 6 hours. Inflammation or edema that occurs due to the administration of carrageenan induction that reaches a maximum of 3-5 hours after induction. Calculated percentage of inflammation from the results of measurement of the volume of mice in each treatment group.
Table 2. Average Edema Foot Volume Measurements
Edema Volume Measurement Average Squeaky Feet (ml)
|
Group |
1 |
2 |
3 |
4 |
5 |
6 |
|
Hr |
Hr |
Hr |
Hr |
Hr |
Hr | |
|
Formula |
0,1 |
0,1 |
0,1 |
0,1 |
0,1 |
0,11 |
|
I |
45 |
42 |
38 |
30 |
25 |
7 |
|
Formula |
0,1 |
0,1 |
0,1 |
0,1 |
0,1 |
0,11 |
|
II |
55 |
50 |
44 |
36 |
20 |
5 |
|
Formula |
0,1 |
0,1 |
0,1 |
0,1 |
0,1 |
0,13 |
|
III |
65 |
61 |
56 |
50 |
47 |
9 |
|
Negative |
0,1 |
0,1 |
0,1 |
0,1 |
0,1 |
0,12 |
|
Control |
50 |
50 |
43 |
39 |
31 |
4 |
|
Positive |
0,1 |
0,1 |
0,1 |
0,1 |
0,1 |
0,12 |
|
Control |
40 |
40 |
37 |
31 |
26 |
0 |
A graph of the average percentage of inflammatory volume of all treatment groups can be seen in Figure 1. According to the graph of the average percentage of inflammation obtained in this study, the negative control group had the highest average percentage of inflammation. This shows that the Formula I, Formula II and Formula III groups have an effect that can reduce edema. When compared to positive controls, the Formula I, Formula II and Formula III groups have a greater
percentage value. This shows that the effect of decreased volume in the Formula I, Formula II and Formula III groups is still smaller when compared to positive controls.
0
100
80
60
40
20
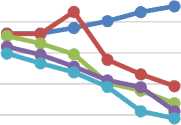

—•— Negative Control
→- Positive Control
—•— Formula I
—•—Formula II
Figure 1 Graph Of Average Percentage of Inflammation of the Feet Squeaking
All the treatment groups can show an anti-inflammatory effect, where the average percentage of edema in each treatment group decreases in the volume of edema. In the formula I, the treatment group, namely the cream base containing 2 g/kg BB pegagan leaves extract, formula II is a cream base containing 4 g/kg BB and formula III which is a cream base containing 6 g/kg BB can be seen at the 1st to 4th hour experiencing a decrease of percent edema. In the negative control treatment that uses pegagan leaves extract 2 g/kg BB can be seen at the 1st hour and the hour to 2 percent edema stable and increase in the 3rd to 6th hour. In the positive control treatment using Betason cream 0.1% can be seen at the 1st hour and the 2% hour edema stable and increased at the 3rd to 6th hour.
DISCUSSION
When viewed from the results of the data in table 3, the entire treatment group occurs inhibition of edema formation on the legs of mice every hour. The formula I treatment group had the maximum effect of inhibition occurring at the 6th hour with a percentage of 69.41%. The formula II treatment group had the maximum effect of inhibition occurring at the 6th hour with a percentage of 77.36%.
Tabel 2. Average Percentage of Volume Inhibition
|
Group |
Average Percentage of Volume Inhibition Squeaky Feet (%) | |||||
|
1 Hr |
2 Hr |
3 Hr |
4 Hr |
5 Hr |
6 Hr | |
|
Negative Control |
0 |
0 |
0 |
0 |
0 |
0 |
|
Positive |
0,2 |
0,2 |
10, |
30, |
46, |
57, |
|
Control |
6 |
6 |
01 |
91 |
52 |
03 |
|
Formula |
2,2 |
8,7 |
22, |
49, |
58, |
69, |
|
I |
3 |
6 |
22 |
47 |
61 |
41 |
|
Formula |
11, |
18, |
32, |
47, |
56, |
77, |
|
II |
74 |
95 |
82 |
52 |
08 |
36 |
|
Formula |
17, |
25, |
37, |
52, |
73, |
80, |
|
III |
71 |
15 |
62 |
58 |
90 |
2 |
The formula III treatment group had the maximum effect of inhibition occurring at the 6th hour with a percentage of 80.2%. The addition of concentration can indicate an increase in anti-inflammatory activity.
The increase in anti-inflammatory activity was influenced by the presence of active substances contained in pegagan leaves extract. After the screening of phytochemicals, pegagan leaves extract contains flavonoids which have properties as anti-inflammatory. Flavonoid compounds can specifically stop the formation and production of substances that cause inflammation resulting from swelling or allergic reactions. The antiinflammatory mechanisms resulting from flavonoids due to inhibition of cox enzyme activity and lipooxygenase can directly lead to inhibition of prostaglandin biosynthesis and leukotrin which are the end products of the COX pathway and lipooxygenase.
Data that has been obtained from the percentage of inflammation will be analyzed using the ANOVA test one way to see the meaning or not of each treatment group. In the ANOVA test must meet the normality and homogeneity of the data. Conducted a normality test using Smirnov's Kolmogorov method to look at the distribution of the percentage data of inflammation of the mice at 1 to 6 o'clock during the study of all normal distributed
treatment groups with a significance of p 0.200 (p>0.05). Test of Homogeneity of Variance results obtained a significance value p 0.001 (p<0.05) which indicates the data variant obtained is heterogeneous. Then continued the one-way ANOVA test with a significance value p 0.012 (p<0.005) which means the effect is significant. The results of the analysis of data from the percentage of inhibition of edema cream extract of pegagan leaves from the treatment group NegativeFormula II and Negative Control-Formula III showed significant difference (p<0.05).
CONCLUSION
Preparation of ethanol extract cream 70% of pegagan leaves with concentrations of 2 g / kg BB, 4 g / kg BB and 6 g / kg BB has an anti-inflammatory effect on edema on the soles of the feet of mice induced carrageenan, with a percentage value of anti-inflammatory power of 69.41% each; 77,36%; 80,2%.
CONFLICT OF INTEREST
There is no conflict of interest in the preparation of this article. This article was written independently by the author.
ACKNOWLEDGEMENT
The authors would like to thank all parts that support this research.
REFERENCES
-
1. Hutapea, J.R. Inventory of medicinal plants Indonesia edition I. Jakarta: Department of the Republic of Indonesia. 1992.
-
2. Yu, H., Y. Ying, X. Fu, H. Lu. Quality determination of Chinese rice wine based on Fourier transform near infrared spectroscopy. Near Infrared Spectrosc. 2006; 14(1) : 37-44.
-
3. Pramono & Ajiastuti. Standardization of Herbal Pegagan Extract(Centella asitica (L.) Urban) Based on Asiticoside Levels in
KLTDensitometri, Indonesian
Pharmaceutical Magazine. 2004; 15(3), 118-123.
-
4. Kurnawan, E. The effect of a combination ointment of wake-up leaf extract (Coleus amboinicus L.) and pegagan leaves herbal extract(Centella asiatica (L.)Urban) on excision wound healing in aloksant-induced
hyperglycemia mice. Student Journal of Faculty of Medicine Untan. 2014; 3(1), 1-19
-
5. Kumar, V., Abbas, K.A., Fausston, N., and Aster, J.C., Robin & Cotran. Pathologic Basic of Disease, 8th edition. Saunders Elsevier Inc., Philadelphia, USA. 2010.
-
6. Nogrady, T. Medicinal Chemistry: Biochemical Approaches. Bandung. ITB. 1992.
-
7. Ditjen POM. Parameter Standar Umum Ekstrak Tumbuhan Obat. DepKes RI, Jakarta. 2000; 30-31.
-
8. Depkes RI. Farmakope Indonesia, Edisi III, Jakarta : Departemen
Kesehatan RI. 1979.
-
9. Setyaningrum, Hesti Dwi dan Cahyo Saparinto. Jahe. Penebar Swadaya. Jakarta. 2013.
-
10. Tranggano, R.I. dan Latifah, F. Buku Pengantar Ilmu Kosmetik. Jakarta: Gramedia Pustaka Utama. 2007.
-
11. GragA. et al., Spreading of Semisolid Formulation: An Update,
Pharmaceutical Technology. 2002: 84102.
-
12. Rowe, C.R., Sheskey, J.P., Weller, J.W. Handbook of Pharmaceutical Excipients. 4th edition. Pharmaceutical Press and American Phamaceu. 2003; 101-103.
18
Discussion and feedback