FORMULATION EFFERVESCENT TABLETS ETHANOL EXTRACT OF PAPAYA LEAVES (Carica papaya L.) AS TOPICAL ANTISEPTIC WITH CITRIC ACID-TARTARIC ACID VARIATIONS
on

Journal of Pharmaceutical Science and Application
Volume 4, Issue 1, Page 1-11, June 2022
E-ISSN: 2301-7708
FORMULATION EFFERVESCENT TABLETS ETHANOL EXTRACT
OF PAPAYA LEAVES (Carica papaya L.) AS TOPICAL ANTISEPTIC
WITH CITRIC ACID-TARTARIC ACID VARIATIONS
Gusti Ayu Nyoman Tri Isa Partama1*, Ni Kadek Ari Kristiani1, Anak Agung Istri Manik Priastari1, I Gusti Ngurah Jemmy Anton Prasetia1
1Department of Pharmacy, Faculty of Math and Science, Udayana University
Corresponding author email: isapartama16@gmail.com
ABSTRACT
Background: Antiseptic products that are widely found in the market contain chloroxylenol which can cause irritation and allergic reactions when used frequently. In addition, antiseptic products in liquid form are considered less practical and economical. The alternative used to overcome these problems is to utilize natural ingredients and are made in solid dosage forms in the form of effervescent tablets. One alternative plant that has the potential as an antiseptic is papaya leaves. Objective: To determine the effect of variations in citric acid and tartaric acid as a source of acid in the formulation of effervescent tablets and to find out what formula is the effervescent tablet of papaya leaf ethanol extract which has the best physical properties to meet the physical qualification requirements of the tablet. Methods: Extraction was carried out by the maceration method with 96% ethanol as solvent. The method used for the manufacture of effervescent tablets is wet granulation. Therefore, in this study, observations were made on the effect of variations in citric acid and tartaric acid (1:3; 1:1 and 3:1) on the physical quality and antibacterial activity of effervescent tablets. Result: The tablet formulations F1 with variation citric acid-tartaric acid (1:3) showed physical characteristics that met the tablet quality requirements. Based on the antibacterial activity test of Escherichia coli on effervescent tablets, papaya leaf ethanol extract (Carica papaya L.) for formulation I showed an inhibitory power of 7,5±1,29 mm. The inhibitory power is more effective than the positive control, namely antiseptic products on the market with an inhibition zone of 6,5 ± 0,05 mm. Conclusion: Based on the variation of citric acid and tartaric acid used, it did not affect the physical properties of effervescent tablets of papaya leaf ethanol extract (Carica papaya L.) including uniformity of weight, but influenced hardness, friability, and dissolving time of effervescent tablets of papaya leaf ethanol extract (Carica papaya L.).
Keywords: Papaya leaf (Carica papaya L.), effervescent tablet, citric acid, tartaric acid, antiseptic
INTRODUCTION
Antiseptic products that are widely found on the market contain chloroxylenol which can cause irritation and allergic reactions when used frequently [8]. This liquid product is considered less practical because it is prone to spills when carried on a trip, so it is not economical. An alternative that can be done to overcome this problem is to utilize natural ingredients that do not cause irritation and allergic reactions which
are made in solid dosage forms in the form of effervescent tablets which are practical because they are easy to use, quickly dissolve in water. And have a longer shelf life [15].
One alternative plant that has the potential as an antiseptic is papaya leaf (Carica papaya L.). Papaya leaves contain antiseptic compounds such as: alkaloids carpainin, carpain, pseudocarpain, vitamins C and E, polyphenols, glycosides, 1
flavonoids, saponins and tannins [9]. Based on Vindi's research [20], the ethanolic extract of papaya leaves (Carica papaya L.) showed inhibition against Staphylococcus aureus bacteria with an inhibition zone diameter of 13,7 mm at a concentration of 20% and an inhibition zone on Escherichia coli bacteria of 12,4 mm at a concentration of 20%.
Based on the content of potentially antiseptic compounds found in papaya leaves (Carica papaya L.), researchers are interested in formulating the potential for ethanol extract of papaya leaves (Carica papaya L.) as a topical antiseptic for effervescent tablets. Effervescent tablet preparations contain acids and bases, which react quickly when added to water to release carbon dioxide gas [19]. When acid and base sources are used in the concentration range of 25-40% of the tablet weight, it can produce good effervescent qualities. According to Anova et al [2], the component that plays a role in the success of making effervescent tablets is the use of variations of citric acid and tartaric acid. Fluctuations in the content of citric acid and tartaric acid significantly affect the physical properties of effervescent tablets [3]. Therefore, researchers are interested in studying the formulation of effervescent tablets made from ethanolic extract of papaya leaves (Carica papaya L.) as a topical antiseptic with varying amounts of citric acid-tartric acid.
METHOD
The material used in this study was papaya leaves taken from the village of Riang Gede Kerambitan, Tabanan Regency. Identification of the actual plant identity at the Eka Karya Bali-LIPI Plant Conservation Research Institute and Botanical Gardens has been declared and the plant used in this study is Papaya Leaf (Carica papaya L.). Caricaceae family. Polyvinyl pyrrolidone (PVP) (Merck), citric acid (Merck), tartaric acid (Merck),
sodium bicarbonate (Merck), lactose (Merck), magnesium stearate (Merck), talc (Merck), ethanol 96% (Merck), aquadest, NA medium, Dettol® antiseptic, Escherichia coli culture.
The equipment used in this study was an oven, water bath, rotary evaporator, blender, analytical balance, glass, digital stopwatch, hardness tester, friability tester, flowability tester, bulk density tester, moisture balance analyzer, mesh filter no. 10, 18, 20, 44, tablet press, caliper,
incubator.
This research was conducted at the Laboratory of non-sterile dosage technology formulation and the Microbiology Laboratory, Faculty of Mathematics and Natural Sciences, Udayana University. All stages of the research were carried out with due observance of the COVID-19 health protocol.
-
3. Preparation and Standardization of Papaya Leaf Simplicia (Carica papaya L.)
The sample used in this study was fresh papaya leaves (Carica papaya L.) aged ± 3 months with finger bones and dark green in color. First, a wet sorting is carried out and then washed to remove dirt for further drainage. After that, it was chopped to speed up the drying process. 10 kg of papaya leaf samples wes dried using an oven at a temperature of 50-60oC for ± 25 hours. The dried simplicia was then blended and sieved using a mesh sieve no. 18 to powder form [20]. Determination of the water content of simplicia was carried out by the gravimetric method according to the Indonesian Pharmacopoeia Edition IV.
-
4. Preparation and Standardization of Papaya Leaf Extract (Carica papaya L.)
A total of 500 grams of papaya leaf Simplicia powder (Carica papaya L.) was
extracted by the maceration method with 5 L 96% ethanol for one day. After that, filtering is carried out. The pulp was macerated again with 3,75 L of 96% ethanol for one day and filtered again. The filtrate obtained was then evaporated with a vacuum rotary evaporator at a temperature of 60oC with a speed and pressure of 70 rpm and 0,7 bars, respectively, to obtain an extract with a thick consistency. After obtaining a thick extract, the yield was calculated [16]. Furthermore, the extract was tested for water content by the gravimetric method in accordance with the Indonesian Pharmacopoeia Edition IV.
-
5. Test of Flavonoid Content in Ethanol Extract of Papaya Leaves (Carica papaya L.)
The flavonoid content test in the extract was carried out by inserting 1 g of thick extract into a test tube, adding 5 ml of ethanol, and then heating it for five minutes in a water bath. Then added 2 drops of concentrated 2N HCL. Then added 0,2 g of Mg powder. Positive results containing flavonoids are indicated by the appearance of a red or orange color layer [20].
-
6. Formulation and Preparation of Effervescent Tablets
Tabel 1. Papaya leaf extract effervescent tablet formulation
|
Material |
Formula (mg) | ||
|
F1 |
F2 |
F3 | |
|
Extract papaya |
100 |
100 |
100 |
|
leaf | |||
|
Citric acid |
31,25 |
62,50 |
93,75 |
|
Tartaric acid |
93,75 |
62,50 |
31,25 |
|
Na bicarbonate |
125 |
125 |
125 |
|
Lactose |
100 |
100 |
100 |
|
Mg stearate |
5 |
5 |
5 |
|
PVP K30 |
20 |
20 |
20 |
|
Talc |
25 |
25 |
25 |
|
TOTAL |
500 |
500 |
500 |
The method used in the formulation of effervescent tablets is the wet granulation method. Granulation was carried out on papaya leaf extract (Carica papaya L.).
Sodium bicarbonate, lactose, citric acid, and tartaric acid, using polyvinyl pyrrolidone K30 as a binder to form a granule mass. After that, it was sifted while the granule mass was wet with a mesh sieve no.10, then dried in an oven at a temperature of 40oC-60oC for 24 hours and sieved again with a mesh sieve no. 20. The dried granules are mixed with talc and magnesium stearate, then the physical quality of the granules is evaluated, after that it is printed with a tablet machine and the physical quality of the tablets is evaluated.
Weigh 50 grams of granules and place them in a flow ability tester. The mass is allowed to flow, and the time is recorded with a digital stopwatch. The requirement for a good flow time is not more than 10 grams/second [11].
-
b. Recess Angle Test
Granule mass as much as 50 grams, placed on the flow ability tester. The mass is allowed to flow. The heap of powder formed was measured in height and radius, calculated using the angle of repose formula. Angle of repose 30o-40o indicates good flow properties [5].
-
c. Compressibility Test
Pour the mass into a 50 mL measuring cup mounted on the bulk density tester and record the initial volume. Turn on the instrument and give it a beat. After that record the final volume. If the compressibility index is in the range of 1115%, this indicates good compression characteristics [5].
-
d. Moisture Test
The moisture content of the particles was tested with a moisture balance analyzer by placing the sample in a container. A good granule moisture content for the manufacture of effervescent tablets is not more than 2-4% [13].
-
8. Evaluation of Effervescent Tablets a. Tablet Organoleptic Test
Visually observed effervescent tablets include uniformity of size, shape, and color of the resulting tablet [4].
-
b. Weight Uniformity Test
A total of 20 tablets were weighed one by one with an analytical balance carefully. Those who meet the requirements if not more, than 2 tablets, each of which weighs 5% deviate from the average weight, and none of the tablets whose weight deviates by 10% from the average weight [6].
-
c. Tablet Hardness Test
The tablet hardness test was carried out using a Hardness tester. The ideal hardness ranges from 4-10 kg/cm2 [18].
-
d. Tablet Friability Test
Take 20 effervescent tablets that have been free of dust and then weighed. The tablet is placed into the friability tester. Ideal fragility if it has a weight loss of <1% [19] .
-
e. Dissolve Time Test
One effervescent tablet was dipped into a beaker glass to which 1000 ml of distilled water had been added. Then calculate the time required by the tablet to dissolve completely. The ideal dissolution time is when it dissolves within 60-120 seconds [14].
Antibacterial activity test using well diffusion method. Add 100 microns of bacterial culture to the NA medium and smooth with a spreading rod. The media is perforated with a cork punch. Then to each well, a sample of the test substance was added in the form of effervescent tablet formulation I, stored at 37°C for 24 hours, and the diameter of the inhibition zone was measured using a caliper. The diameter of the inhibition zone was compared with the positive control (Dettol® antiseptic) and negative control (aquadest) [20].
The test results were statistically analyzed using OneWay ANOVA with a
95% confidence level. In addition, the LSD test (Least Significance Different) was performed to explain the differences between each formula in the physical properties of the tablets in the test. In addition, the results obtained were compared with the Indonesian Pharmacopoeia and other literature.
RESULT
-
1. Results of Water Content Testing of Papaya Leaf Simplicia (Carica
papaya L.)
Based on the results of testing the water content of papaya leaf simplicia (Carica papaya L.) obtained 4,48±0,67%.
-
2. Flavonoid Test Results on Papaya Leaf Extract (Carica papaya L.) Phytochemical screening test results in the ethanol extract of papaya leaves (Carica papaya L.) contain flavonoid compounds. The results of the phytochemical screening test were marked by the formation of a reddish-orange layer which can be seen in Figure 1.

Figure 1. A reddish-orange layer is formed
-
3. Effervescent Granule Evaluation Results
Table 2. Results of Effervescent
Granules of Rest Angle Testing
|
Effervescent Granule Evaluation |
Formula | ||
|
F1 |
F2 |
F3 | |
|
Flow Time (s) |
3,41 |
3,13 |
3,32 |
|
Angle of Repose (o) |
37,35 |
31,24 |
35,10 |
|
Compressibility |
12,66 |
14,66 |
12,66 |
|
(%) Moisture Content |
2,75 |
2,82 |
2,52 |
|
(%) | |||
4. Evaluation Results of Effervescent
Tablets

Figure 2. Organoleptic test of effervescent tablets
Table 6. Test Results of Effervescent
Tablets Dissolving Time
|
Formula |
Average Dissolution Time of Effervescent Tablet(s) |
|
F1 |
71,80±1,09 |
|
F2 |
102,80±19,30 |
|
F3 |
199,80±34,47 |
The effervescent tablet solution sample used was formulation I, this formulation was chosen because F1, F2 and F3 contained the same extract concentration, which was 20% of the tablet weight. These three formulations contain ethanol extract of papaya leaves (Carica papaya L.) which has antibacterial activity against Escherichia coli bacteria. The results of the diameter of the inhibition zone of papaya leaf ethanol extract (Carica papaya L.) against Escherichia coli bacteria can be seen in Table 7.
Table 3. Calculation Results of Average Weight Uniformity
|
Formula |
Average weight (g) |
Deviation of mean weight (5%) effervescent tablet (g) |
|
F1 |
0,503 |
0,478 - 0,529 |
|
F2 |
0,504 |
0,479 - 0,529 |
|
F3 |
0,506 |
0,480 - 0,531 |
Table 7. Result of Inhibitory Zone Diameter of Escherichia coli.
|
Sample |
Inhibition zone diameter (mm) (Average±SD) |
|
Papaya leaf extract effervescent tablet |
7,5±1,29 |
|
solution (Formulation I) Control (+) |
6,5±0,05 |
|
Control (-) |
0 |
Table 4. Results of Hardness Testing of
Effervescent Tablets
|
Formula |
Average Hardness of Effervescent Tablets (Kg/cm2) |
|
F1 |
8,49±0,52 |
|
F2 |
6,15±0,59 |
|
F3 |
3,49±0,62 |
Table 5. Effervescent Tablet Friability Test Results
|
Formula |
Average Friability of Effervescent Tablets (%) |
|
F1 |
0,23±0,02 |
|
F2 |
0,25±0,01 |
|
F3 |
1,20±0,01 |
DISCUSSION
This research was made in the effervescent tablet formula of papaya leaf extract (Carica papaya L.) by varying the amount of citric acid-tartric acid (1:3), (1:1), (3:1). This treatment is expected to produce physical quality characters of effervescent tablets that meet the requirements.
Samples of papaya leaves (Carica papaya L.) obtained as much as 10 kg were dried using an oven at a temperature of 50oC-60oC for ± 25 hours. Dried simplicia obtained weight of 5 kg. The results of the 5
simplicia water content test were 4,48 ± 0,67%. These results have met the requirements of the simplicia quality standard, which is <10%. Simplicia with low water content will prevent the growth of microorganisms that make simplicia become damaged [20]. The papaya leaf simplicia powder obtained was extracted by the maceration method as much as 500 grams of simplicia using 96% ethanol as solvent. Then the filtrate was evaporated using a rotary evaporator. Evaporation aims to separate the solvent to produce an extract with a more concentrated content [16]. The weight of the thick extract was 44,10 grams with a yield of 8,82%. The results of the analysis of the ethanol extract of papaya leaves (Carica papaya L.) were positive for flavonoid compounds. The papaya leaf extract used was dissolved in ethanol and then heated. Heating is done because most of the flavonoid compounds can dissolve when heated. The results obtained were the formation of a red layer after the addition of HCI and Mg powder. The resulting orange-red layer indicates the presence of flavonoid compounds from the reduction of concentrated hydrochloric acid and magnesium [20]. This can be seen in Figure 1.
Papaya leaf extract (Carica papaya L.) was then formulated into effervescent granules with the addition of non-toxic, inert, physically, and chemically stable additives. The additional material used is lactose as a filler which functions to regulate the weight of the tablet so that it is easy to compress. Lactose has good watersolubility properties. Polyvinyl pyrrolidone (PVP) as a binder is generally used in the manufacture of effervescent tablets. This is because the solubility of PVP is good, PVP-alcohol is used to improve good
granulation, dries quickly, and has good compression properties [10]. Magnesium stearate as lubricant and talc as glidants. The combination of talc and magnesium stearate produces three advantages, namely as a print separator that avoids the sticking of the tablet mass, as a lubricant to facilitate tablet dispensing, and reduces friction between the walls of the printing chamber and the side surface. Tablets, and as a flow regulator to increase mass glide or granule flow time. While the acid and base sources produce a good effervescent reaction when used in the concentration range of 25%-40% of the tablet weight [2].
Granulation formation is a process of increasing particle size that coalesces into larger aggregates (clots) and has good flowability when printed [19]. Good granules must meet the physical requirements of granule evaluation with the aim of maximizing performance in the effervescent tablet molding process. Evaluation of the granules carried out included: the flow time test, angle of repose test, compressibility test, and water content test [7].
The granule flow time test aims to ensure good flow properties of the granule powder so that it will be efficient to be printed on the machine. The flow properties are influenced by the size and shape of the particles. Larger and rounder particles indicate good flow properties [19]. In Table 2. The results of the granule flow time test have met the quality requirements of the granule flow time, which is not more than 10 grams/second [11]. Based on the results of the Oneway ANOVA analysis showed that there was no difference in the average flow time in the different formula groups (p>0.05). It can be interpreted that variations in the concentration of citric and
tartaric acids do not affect the flow time value.
The granule angle of repose test aims to determine the angle between the coneshaped particle group and the horizontal plane. When using a measuring device to pour a certain amount of powder. The size of the angle of repose is influenced by the shape, size, and humidity of the particles [13]. In Table 2. The test results of the angle of repose of the three formulations have met the requirements, which are between 30o-40o which shows they have good flow properties [5]. Based on the results of the Oneway ANOVA analysis, it was shown that there were differences in the average angle of repose in the groups of different formulas (p<0.05). It can be interpreted that variations in the amount of citric acid and tartaric acid affect the angle of repose. The angle of repose describes the cohesion and friction between particles. From Table 2 the use of variants of citric acid and tartaric acid increases the angle of repose of the particles. This is due to the greater cohesion between the citric acid and tartaric acid particles, which results in a smaller angle of repose. The mixture of the two acid variants reduces the cohesion of the particles, thereby increasing the angle of repose [13].
The granule compressibility test aims to determine the ability of granule density and decrease in granule volume due to shock. In Table 2. The results of the compressibility test of the three formulations have met the requirements of a good compressibility index of 11%-15% [5]. Based on the results of the Oneway ANOVA analysis, it showed that there were differences in the average compressibility of the different formula groups (p<0.05). It can be interpreted that variations in the amount of citric acid and tartaric acid affect
the compressibility test. This is due to the uneven shape and particle size of the granules from the three different formulations [14].
Moisture content testing aims to determine the moisture content and moisture contained in a granule. In Table 2. The results of the moisture content test of the three formulations have met the requirements for a good moisture content of effervescent granules, which is not more than 2-4% [13]. Based on the results of the Oneway ANOVA analysis, it showed that there were differences in the average moisture content of the different formula groups (p<0.05). It can be interpreted that variations in the amount of citric acid and tartaric acid affect the granule moisture content test. This is because the room where the granules are produced has a relatively high humidity of 55-60% which makes citric acid easily hygroscopic. while the relative humidity of the effervescent granule production room is 25% [17].
The homogeneous granules that have met the requirements for the effervescent granule test are then printed with a tablet press machine and tested for evaluating the quality of physical preparations of effervescent tablets which include organoleptic test, and weight uniformity test, tablet hardness test, tablet friability test and tablet dissolving time test.
Organoleptic test of effervescent tablets based on the observations in Figure 2, it can be concluded that the physical appearance of effervescent tablets has a uniform size, round shape with a flat surface, and a characteristic odor of papaya leaves, and is slightly dark green in color.
In testing the uniformity of tablet weights. Tablets are printed with an expected average weight of 500 mg, based 7
on the requirements of the third edition of the Indonesian Pharmacopoeia that there should be no more than two tablets whose weights deviate by 5% (475 mg-525 mg) from the average weight, and one of the tablets weighed 10% (450 mg-550 mg) from the average weight. Based on Table 3. it can be concluded that the three tablet formulas meet the quality requirements for weight uniformity because they do not exceed the requirements according to Pharmacopoeia Edition III [6]. Based on the results of the Oneway ANOVA analysis that there was no difference in the average uniformity of tablet weights in the three formula groups (p>0.05).
In the tablet hardness test in Table 4. it can be concluded that F1 and F2 already meet the tablet hardness standard because the tablet hardness values are in the range of 4-10 kg/cm2 [18]. Meanwhile, F3 has a tablet hardness of less than 4 kg/cm2 so it does not meet the quality standard for effervescent tablet hardness [1]. Based on the results of the Oneway ANOVA analysis that there is a difference in the average tablet hardness in the three groups of formulas (p<0.05). It can be interpreted that variations in citric and tartaric acids affect tablet hardness. The more citric acid added to the formula, the lower the tablet hardness value. This is because citric acid is hygroscopic, which can increase the water content of the granules and make the tablets softer [22].
In the tablet friability test in Table 5. it can be concluded that F1 and F2 have met the tablet friability requirements because they have a percentage of friability value of less than 1% [19]. Meanwhile, F3 did not meet the tablet friability quality requirements. Based on the results of the Oneway ANOVA analysis that there is a
difference in the average tablet hardness in the three groups of formulas (p<0.05). Hardness is another description of tablet brittleness which is related to the bond strength of the particles at the edges and surface of the tablet. If the hardness of a tablet is low, it will have an impact on the low friability of the tablet [4].
In testing the dissolution time of effervescent tablets in Table 6. it can be concluded that F1 and F2 have met the quality standard of the ideal effervescent tablet dissolving time test, which dissolves within 1-2 minutes [14]. Meanwhile, F3 did not meet the quality standard of effervescent tablet dissolving time. Based on the results of the Oneway ANOVA analysis that there is a difference in the average dissolving time in the three groups of formulas (p<0.05). Temperature and humidity on storage greater than 25% influence the dissolution rate of an effervescent tablet. The presence of a lot of water vapor triggers the effervescing reaction so that when the tablet dissolves, the reaction between the acid and base components is very slow. This causes the tablet to dissolve longer [10].
The antibacterial activity test of effervescent tablets of papaya leaf ethanol extract (Carica papaya L.) against Escherichia coli bacteria was carried out using the well diffusion method. In this study, samples were made by dissolving effervescent tablets of formula I which contained 20% ethanol extract of papaya leaves (Carica papaya L.). The negative control used was aquadest to see if the solvent used had antibacterial activity which could later bias the results of the study. While the positive control used was antiseptic preparations containing
chloroxylenol which were widely circulated in the market.
The results of the antibacterial activity test for the effervescent solution sample of formula I in Figure 3. show that the effervescent tablet preparation of papaya leaf ethanol extract (Carica papaya L.) made can inhibit the growth of Escherichia coli bacteria which is characterized by the formation of an inhibition zone around the well. The results of the antibacterial activity test of the preparation can be seen in Table 7.
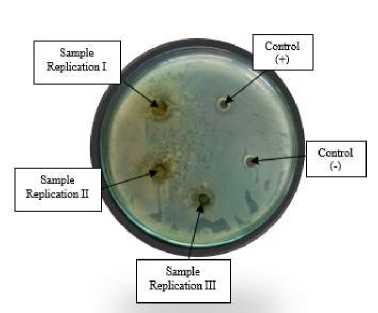
Figure 3. The results of the antibacterial activity of papaya leaf ethanol extract of effervescent tablets against Escherichia coli bacteria
Based on the test results, it can be concluded that the result of the negative control inhibition zone is 0 mm. This shows that the aquadest solvent used does not have an antibacterial activity which is indicated by the absence of a clear zone around the well, so it does not affect the results of the antibacterial test. The positive control had an average inhibition zone of 6,5±0,05 mm, which means it was in the category of medium inhibition zone. The results of the antibacterial test of the effervescent tablet formulation I containing 20% ethanol extract of papaya leaves (Carica papaya L.) showed that the diameter of the resulting inhibition zone was 7,5±1,29 mm which was included in the medium category.
However, this inhibition was more effective when compared to positive control. This shows that the papaya leaf extract (Carica papaya L.) contained in the sample has an active compound as an antibacterial. Based on the phytochemical results. Papaya leaves (Carica papaya L.) contain high amounts of flavonoid compounds. Flavonoids are secondary metabolites that act as antimicrobials and antivirals. The flavonoid compounds contained in the papaya leaf extract function as type II topoisomerase inhibitors that can inhibit bacterial DNA replication and transcription and can bind extracellular bacterial proteins, and dissolve bacterial cell walls [16].
CONCLUSION
Based on the variation of citric acid and tartaric acid used, it did not affect the physical properties of effervescent tablets of papaya leaf ethanol extract (Carica papaya L.) including uniformity of weight but influenced hardness, friability, and dissolving time of effervescent tablets of papaya leaf ethanol extract (Carica papaya L.). Effervescent tablet formulations that meet the physical qualification requirements are F1 with variation citric acid-tartaric acid (1:3). Based on the antibacterial activity test of Escherichia coli on effervescent tablets, papaya leaf ethanol extract (Carica papaya L.) for formulation I showed an inhibitory power of 7,5±1,29 mm. The inhibitory power is more effective than the positive control, namely antiseptic products on the market with an inhibition zone diameter of 6,5±0,05 mm.
CONFLICT OF INTEREST
There is no conflict of interest in the preparation of this article. This article was written independently by the author without the involvement of any third party or party.
ACKNOWLEDGEMENT
The authors would like to thank the Ministry of Education, Culture, Research,
and Technology of the Republic of Indonesia and the Chancellor of Udayana University through the Pharmacy Study Program, Faculty of Mathematics and Natural Sciences, Udayana University for facilitating and funding this research.
REFERENCE
-
1. Ansel H. Penghantar Bentuk Sediaan Farmasi. 4th edition. Jakarta: UI Press; 1989. pp. 132.
-
2. Anova I, T Hermianti W, Kamsina. Formulasi Perbandingan Asam Basa Serbuk Effervescent dari Coklat Bubuk. Jurnal Litbang Industri. 2016; 6 (1): 99- 106.
-
3. Anwar K. Formulasi Sediaan Tablet Effervescent dari Ekstrak Kunyit (Curcuma domestica Val.) dengan Variasi Jumlah Asam Sitrat-Asam Tartrat Sebagai Sumber Asam. Jurnal Sains dan Terapan Kimia. 2016; 4(1): 168-178.
-
4. Apriani N P, Arisanti C I S. Pengaruh Penggunaan Amilum Jagung
Pregelatinasi Sebagai Bahan Pengikat Terhadap Sifat Fisik Tablet Vitamin E. Jurnal Farmasi Udayana. 2014; 3(2), 59-63.
-
5. Aulton M. Pharmaceutical: The
Science of Dosage Form Design. 2nd edition. Endiburgh: Churchill
Livingstone; 2002.
-
6. Depkes RI. Farmakope Indonesia Edisi III. Jakarta: Departemen Kesehatan Republik Indonesia; 1979.
-
7. Depkes RI. Farmakope Indonesia Edisi
-
IV. Jakarta: Departemen Kesehatan Republik Indonesia; 1995.
-
8. Jasmine. Perbandingan Efek
Pemakaian Antiseptik Chloroxylenol 4,8% dan Povidone Iodine 7,5% Terhadap Jumlah Koloni Bakteri Pasca Pencucian Tangan Rutin WHO Mahasiswa Kepaniteraan Klinik di Departamen Bedah Mulut FKG USU Periode Maret-Mei 2018. Sumatera Utara: Universitas Sumatera Utara; 2018.
-
9. Jimenez V, M Mora-Newcomer, E, Gutierrez-Soto MV. Biology of the papaya plant. In: Ming R, Moore PH. Genetics, and genomics of papaya. New York. 2014; 17(9): 22-35.
-
10. Kholidah S, Yuliet, Akhmad K. Formulasi Tablet Effervescent Jahe (Z Officinale Roscoe) Dengan Variasi Konsentrasi Sumber Asam dan Basa. Online Jurnal of Natural Science. 2014; 3(3): 216-229.
-
11. Kemenkes RI. Farmakope Indonesia Edisi V. Jakarta: Kementerian
Kesehatan Republik Indonesia; 2014.
-
12. Kartikasari S D, Yosi B, Mufrod. Formulasi Tablet Effervescent Ekstrak Rimpang Jahe Emprit (Zingiber
officinale Rosc.) Dengan Variasi Kadar Asam Sitrat dan Asam Tartrat. Traditional Medicine Journal. 2015; 20(2):119-126.
-
13. Lachman L, Lieberman H. Teori dan Praktek Farmasi Industri. 2nd edition. Jakarta: UI Press; 1994.
-
14. Lachman L, Lieberman H. Teori dan Praktek Farmasi Industri. 3rd edition. Jakarta: UI Press; 2008.
-
15. Lindberg N, Engfors H, Ericson T. Effervescent Pharmaceuticals in Swarbricck, J., Boyland, J.C. (eds). Encyclopedia of Pharmaceutical Technology. Marcell dekker Inc. New York. 1992; 5(1): 45-71.
-
16. Mahatriany N, N Payani, N P S Oka, I B M, Astuti K W. Skrining Fitokimia Ekstrak Etanol Daun Pepaya (Carica papaya L.) yang Diperoleh Dari Daerah Ubud, Kabupaten Gianyar, Bali. Jurnal Farmasi Udayana. 2015; 4(5): 168-201.
-
17. Mohrle R. Effervescent Tablet in Pharmaceutical dosage Form Table. New York: Marcel Dekker Inc; 1989.
-
18. Parrot E. Pharmaceutical Technology. United States of America: Burgess Publishing Company; 1970.
-
19. Siregar Charles JP, Saleh Wikarsa. Teknologi Farmasi Sediaan Tablet
Dasar-Dasar Praktis. Jakarta: Penerbit Buku Kedokteran EGC; 2010.
-
20. Vindi E K. Uji Aktivitas Antibakteri Ekstrak Daun Pepaya (Carica pepaya L.) Terhadap Bakteri Escherichia coli dan Staphylococcus aureus. Jurnal FARMASINDI. 2019; 3(2): 16-20.
-
21. Voigt R. Buku Pedoman Teknologi Farmasi. Edisi Kelima. Yogyakarta: Penerbit Gadjah Mada University Press; 1984.
-
22. Yulianti D A, Suyatno S. (2021). Formulasi Tablet Effervescent Ekstrak Daun Katuk (Sauropus androgynous L. Merr.) dengan Variasi Konsentrasi Asam dan Basa. Journal of Pharmacy Science and Practice. 2021; 8(1):35-39.
11
Discussion and feedback