SYNERGISTIC EFFECT OF CURCUMA XANTHORRHIZA AND PHYSALIS ANGULATA EXTRACTS AS ANTIOXIDANTS AGAINST DPPH RADICALS
on
Journal Pharmaceutical Science and Application
IPCΔ Volume 5, Issue 2, Page 85-92, December 2023
J E-ISSN: 2301-7708
SYNERGISTIC EFFECT OF CURCUMA XANTHORRHIZA AND
PHYSALIS ANGULATA EXTRACTS AS ANTIOXIDANTS AGAINST
DPPH RADICALS
Putu Yudhistira Budhi Setiawan1*, I Putu Gede Adi Purwa Hita1, I Putu Riska Ardinata1, Ni Putu Aryati Suryaningsih1·
1Clinical Pharmacy Study Program, Faculty of Health Sciences, Bali International University
Corresponding author email: yudhistirabudhi@gmail.com
ABSTRACT
Background: As a single extract, Curcuma xanthorrhiza and Physalis angulata are known to have antioxidant activity. However, the interaction effect of antioxidants in suppressing DPPH (2,2-difenil-1-pikrilhidrazil) radicals that arise in these two combinations of plant extracts is not yet known. In this study, the combination of these two plants was investigated for their antioxidant properties and synergistic effects. Objective: This study aims to determine the effect of the combination of Curcuma xanthorrhiza and Physalis angulata as antioxidants and the interactions they cause. Methods: The rhizomes of Curcuma xanthorrhiza and the herb Physalis angulata were extracted separately with ethanol. After evaporation, the crude xtract was tested individually to obtain the IC50 value. Based on the IC50 value of each extract, 9 combination variations were made and the combination index (CI) value was calculated to determine the resulting interaction effect. Result: The antioxidant activity of Curcuma xanthorrhiza IC50 extract is 51.015 ppm, higher than Physalis angulata IC50 extract which is 90.488 ppm. The CI value observed in the combination of extracts in all combinations had a CI value <1.Conclusion: This research shows that the combination of Curcuma xanthorrhiza rhizome extract and Physalis angulata herb has synergistic antioxidant properties.
Keywords: Combination; Synergis; Antioxidant; Combination Index,
INTRODUCTION
The presence of reactive oxygen species (ROS) in cells and tissues causes damage in excessive amounts and is often associated with degenerative diseases[1]. The production and accumulation of ROS or free radicals, which are produced by biochemical reactions in the body, are mediators of many diseases including cancer, rheumatoid arthritis, kidney disease, cardiovascular disease, neurological diseases and respiratory diseases[2]. The body naturally produces endogenous antioxidant molecules, such as
glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), to fight free radicals. If the number of radical molecules exceeds the internal antioxidant capacity, it triggers oxidative stress[3]. This situation requires exogenous antioxidants which will increase the body's antioxidant capacity. Alternatives can be sourced from plants containing bioactive compounds that are able to stabilize free radicals.
Secondary metabolite compounds, especially polyphenols produced by plants,
have antioxidant activity[4]. The existence of oxidation-reduction properties allows phenolic compounds to act as reducing agents, proton donors, free radical scavengers, and peroxide decomposers which cause them to have antioxidant effects[5]. The curcumin compound is a group of polyphenols contained in plants of the curcuma genus[6]. The activity of curcumin is able to reduce inflammation by increasing enzymatic antioxidant activity through increasing methionine sulfoxide reductase A (MSRA) expression and increasing several enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)[7]. Several studies report the potential natural antioxidant activity of Curcuma xanthorrhiza extract has been evaluated using various methods including DPPH (1,1-diphenyl-2-picryhidazi), superoxide anion, iron reducing antioxidant power (FRAP), and metal binding activity[8]. Although the C. xanthorrhiza plant has demonstrated antioxidant properties, clinical trials have shown that the curcumin content has poor bioavailability[9].
The approach to overcome this problem is to combine two or more phytochemicals to obtain a synergistic effect so that there is no need to increase the dose. The efficacy activity of a compound can be increased without drastically increasing the administered dose to avoid possible side effects[10]. One approach to increase antioxidants is by combining it with Physalis angulata extract. The P. angulata plant is often found in tropical areas, containing phenolic and flavonoid compounds that have antioxidant activity[11]. A new phenolic glycoside, physanguloside A, isolated from P. angulata has the activity of suppressing nitric oxide which is a radical gas compound[12]. Extracts with antioxidant properties are combined, a synergistic effect is possible and a stronger antioxidant effect is
produced than the amount produced with a single extract[13].
In this study, the antioxidant activity of the ethanol extract of C. xanthorrhiza (EECX) and the ethanol extract of P. angulata (EEPA) was studied using the DPPH free radical scavenging power. Determination of the synergistic effect of the combination of the two extracts was calculated using the combination index (CI) equation. The aim of this research is to provide scientific evidence that the use of a combination of herbal medicines can increase the antioxidant effect.
METHODS
C. xanthorrhiza rhizomes were collected in Singaraja, while P. angulata herbs were collected in Gianyar. Both districts are in Bali, Indonesia. Both plants were identified in Bali Botany Garden, Indonesian Institute of Sciences. Methanol, DPPH were purchased from Sigma-Aldrich. The solvent used for the extraction process using technical grade ethanol was purchased from CV Genera Labora. The analytical instrument uses a spectrophotometer type 8100 Thermo scientific
-
2. Preparation of Curcuma xanthorrhiza and Physalis angulata extracts
Separately, Physalis angulata herbs and Curcuma xanthorrhiza rhizomes are washed under running water and drained. Both ingredients were dried in an oven for 2 days at 50ºC and made into a fine powder. Powdered plant material is extracted using the maceration method with a ratio of 1 part dry plant powder macerated to 10 part ethanol (70%) for 24 hours. This maceration process is repeated 3 times. At that time, the maceration was concentrated using a rotary evaporator and then continued in a water bath until it became a crude extract.
The DPPH radical scavenging activity test was carried out with a number of samples with a certain concentration added with 1.0 mL of 0.4 mM DPPH and methanol added to 5 ml. The mixture was then vortexed and left for 30 minutes at room temperature, protected from light by wrapping in aluminum foil. The absorbance of the mixture was then measured at the maximum wavelength with the blank used being methanol. The percentage of inhibition was determined by the following formula:
Radical scavenging = ^x^^χ 100%
Information Ax: DPPH control absorbance and As: Sample absorbance. The value (IC50) of each extract was calculated based on the linear regression equation y = bx + a resulting from a plot of sample concentration vs % radical scavenging.
The combined antioxidant effect of the two extracts was calculated based on the IC50 value of each single extract. Each extract was made into a series of solution concentrations, including ¼IC50, ½IC50, and ¾IC50. Three series of concentrations of each extract were then combined to produce a total of 9 combinations. The absorbance of the combined solutions was then measured to obtain the percentage of radical scavenging activity. CI calculations are used to determine whether a combination has a synergistic effect or not. The equation for determining CI is as follows:
CI = — + — Dxl Dx2
Where D1 and D2 are the concentrations of each combined extract that produces effect x, Dx1 and Dx2 are the concentrations of each single extract that produces effect x. A CI
value < 1 indicates a synergistic interaction, a CI = 1 indicates an additive interaction, and a CI > 1 indicates an antagonistic interaction[14].
RESULTS
Each ethanolic extract of Curcuma xanthorrhiza (EECX) and Physalis angulata (EEPA) has activity in scavenging DPPH free radicals. The percentage of free radical inhibitory activity increases with increasing EECX concentration (Figure 1). The antioxidant activity of EEPA also shows the same thing but is not as strong as the activity of EECX (Figure 2). The strength of the two extracts can be seen from the IC50 value where EECX is 51.015 ppm while EEPA is 90.488 ppm.
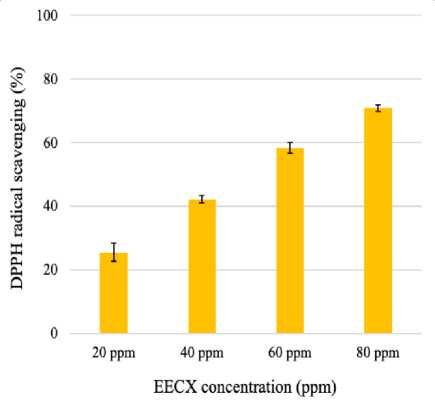
Figure 1. DPPH radical scavenging activity of Curcuma xanthorrhiza (EECX). Error bars represent the standard deviation (n=3).
The amount of free radical inhibitory activity for each combination of EECX and EEPA varies depending on the ratio and concentration of each extract (Figure 3). All combinations plotted on the isobologram all fall into the synergistic area (Figure 4).
Combination number 9 has the highest EECX and EEPA ratio while also having the highest antioxidant activity. On the other hand, combination number 1 has the lowest activity because the concentration ratio is also the smallest. The results of the analysis showed that the CI value of all combinations had a value of less than 1, which means that the combination of EECX and EEPA produced a synergistic effect as an antioxidant against DPPH radicals. The CI value results obtained by combination number 4 (Table 2) with a comparison of the EECX and EEPA ratios of (½ IC50 : ¼ IC50 ) have the smallest value of 0.755.
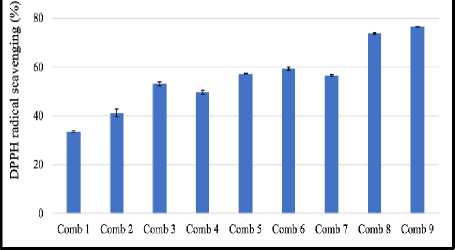
Figure 3. DPPH radical scavenging activity of Combination extract. Error bars represent the standard deviation (n=3).
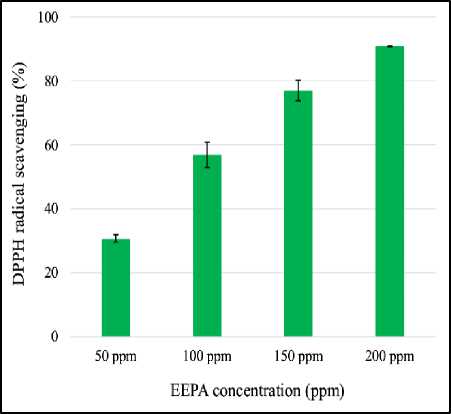
Figure 2. DPPH radical scavenging activity of Physalis angulata (EEPA). Error bars represent the standard deviation (n=3).
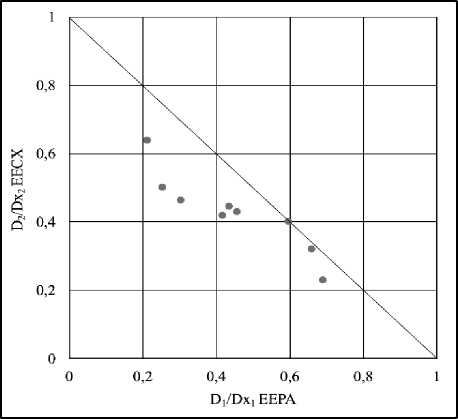
Figure 4. Isobologram of DPPH radical scavenger combination test
Table 1. DPPH radicals scavenging activities of the extracts
|
Sample |
Regression equation |
R2 |
IC50 (ppm) |
Antioxid ant activity |
|
Physalis |
Y=0.4011X |
0.98 |
90.488 |
Strong |
|
angulata |
+13.705 |
1 | ||
|
Curcuma |
Y=0.7631X |
0.99 |
51.015 |
Strong |
|
xanthorrhiza |
+11.07 |
5 |
DISCUSSION
The extraction of both plants uses maceration with 70% ethanol solvent because the method is simple and is able to universally extract compounds and prevent damage to compounds which are thermolabile and not heat resistant[15]. In this study, we have reported the characteristics of the two extracts in the results of previous research[16].
Table 2. The combination index (CI) values of the DPPH radical scavenging activities of the combination extract
|
Ratio |
Sample code |
DPPH radical scavenging | ||
|
C. xanthorrhiza |
P. angulata |
CI |
Remarks | |
|
¼ IC50 |
¼ IC50 |
Comb 1 |
0.886 |
Synergism |
|
¼ IC50 |
½ IC50 |
Comb 2 |
0.981 |
Synergism |
|
¼ IC50 |
¾ IC50 |
Comb 3 |
0.920 |
Synergism |
|
½ IC50 |
¼ IC50 |
Comb 4 |
0.755 |
Synergism |
|
½ IC50 |
½ IC50 |
Comb 5 |
0.837 |
Synergism |
|
½ IC50 |
¾ IC50 |
Comb 6 |
0.997 |
Synergism |
|
¾ IC50 |
¼ IC50 |
Comb 7 |
0.851 |
Synergism |
|
¾ IC50 |
½ IC50 |
Comb 8 |
0.767 |
Synergism |
|
¾ IC50 |
¾ IC50 |
Comb 9 |
0.881 |
Synergism |
The antioxidant activity results from EECX have an IC50 value of 51,015 ppm, with a DPPH radical scavenging test. Measurement of antioxidant activity using the DPPH radical capture method because it is most widely used in measuring antioxidant activity in vitro in medicinal plants[17]. The antioxidant activity of EECX is because it contains curcuminoids and xanthorrizol, there is a correlation between the amount of these compounds and the antioxidant activity produced. The content of these compounds increases with increasing plant age[18]. Curcumin contains in its structure a methoxy group substituted with a hydrogen atom. This functional group is what donates hydrogen atoms when measuring DPPH radical activity. The antioxidant activity of Curcuma xanthorrhiza extract is 32-77 ppm, this difference is due to different growing locations which are external factors. Growing location factors also influence the total phenolic content of Curcuma xanthrorriza extract[19]. Total phenolics determine antioxidant activity, a positive correlation was found between antioxidant activity and phenolic content using correlation coefficients[20].
Comparison of IC50 values between these studies is limited due to differences in methodology in compound quantification, extract production, and types of plant parts studied. The antioxidant activity of EEPA is
quite high at IC50 90.488 ppm compared to previous research. Physalis angulata leaf methanol extract has antioxidant activity but the activity is very weak with an IC50 value of 820.569 ppm [21]. The difference in activity is due to antioxidant testing using the microplate method. The best total phenolic content and DPPH radical scavenging activity were found in the leaves, followed by fruit, roots and stems[11]. Differences in the type of fraction can influence antioxidant activity, previous research shows that the ethyl acetate fraction of the Physalis angulata herb has the highest antioxidant activity with an IC50 value of 213.34 ppm compared to the water fraction, and the n-hexane fraction and the crude extract. TLC identification of the ethyl acetate fraction shows spots that lead to flavonoid compounds having characteristics such as quercetin spots, these compounds are responsible for antioxidant activity [22].
Both extracts have antioxidant activity, where EECX is stronger as seen from the small IC50 value (Table 2). The combination of the two extracts has better antioxidant activity than the use of a single extract. The use of a combination with a ratio of half the IC50 dose of each extract in combination number 5 (Figure 3) is able to provide more than 50 percent inhibition of DPPH radicals. These results show that the two components interact to strengthen
antioxidant activity. The results of the combination test using extracts combined with a ratio comparison based on the IC50 value, produced a synergistic effect in the nine combination variations (Table 3). The interaction between active compounds that act as antioxidants in a combination of extracts is greatly influenced by the concentration and ratio of the bioactive components of the extract[23]. Previous research combined Coccinia grandis and Blumea balsamifera extracts, the interaction at high concentrations and ratios gave an antagonistic effect, while at low concentrations and ratios it gave a synergistic effect[24]. The mechanism for this synergism may be caused by antioxidant regeneration where EEPA which has lower activity is strengthened by the addition of EECX[23]. The increase in combined antioxidant activity is caused by various specific mechanisms, such as self-protection mechanisms because the combined compound is able to simultaneously detect several antioxidant functions and destroy several physiological radical species, inhibit prooxidant apoenzymes, and reduce and chelate iron ions[25].
CONCLUSION
Curcuma xanthorrhiza (EECX) and Physalis angulata (EEPA) individually have antioxidant activity in scavenging DPPH radicals. Combination number 4 has the best synergistic effect with the smallest CI value, the highest DPPH radical inhibitory activity is obtained in combination number 9. The combination of the two extracts is synergistic in all nine concentration ratios, causing an increase in antioxidant capacity.
CONFLICT OF INTEREST
We declare that there was no conflict of interest when preparing this article and it was written independently by the author without the presence of other parties.
ACKNOWLEDGEMENT
The author would like to thank the Chancellor of Bali International University through the Clinical Pharmacy Study Program, Faculty of Health Sciences, Bali International University for facilitating this research.
REFERENCES
-
1. Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry. 2004 Mar 1;84(4):551–62.
-
2. García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Medicine and Cellular Longevity. 2020 Jul 24;2020:1–16.
-
3. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Medicine and Cellular Longevity. 2017;2017:1– 13.
-
4. Luo J, Si H, Jia Z, Liu D. Dietary AntiAging Polyphenols and Potential Mechanisms. Antioxidants. 2021 Feb;10(2):283.
-
5. Olszowy M. What is responsible for
antioxidant properties of polyphenolic compounds from plants? Plant Physiology and Biochemistry. 2019 Nov 1;144:135–43.
-
6. Pulido-Moran M, Moreno-Fernandez J,
Ramirez-Tortosa C, Ramirez-Tortosa Mc. Curcumin and Health. Molecules. 2016 Feb 25;21(3):264.
-
7. Meshkibaf MH, Maleknia M, Noroozi
S. Effect of curcumin on gene expression and protein level of
methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund’s adjuvant inflammation-induced male rats. Journal of Inflammation Research. 2019;12:241.
-
8. Rahmat E, Lee J, Kang Y. Javanese Turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, Phytochemistry, Biotechnology, and Pharmacological Activities. Evid Based Complement Alternat Med. 2021 Jun
11;2021:9960813.
-
9. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of Curcumin: Problems and Promises. Mol Pharmaceutics. 2007 Dec 1;4(6):807–18.
-
10. Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects and
mechanisms of combined
phytochemicals. The Journal of
Nutritional Biochemistry. 2019 Jul 1;69:19–30.
-
11. Ferreira LDSL, Do Vale A, De Souza A, Leite K, Sacramento C, Moreno MV, et al. Anatomical and phytochemical characterization of Physalis angulata L.: A plant with therapeutic potential. Phcog Res. 2019;11(2):171.
-
12. Anh HLT, Dung DT, Tuan DT, Tai BH, Nhiem NX, Yen PH, et al. New Phenolic Glycosides from Physalis angulata. Natural product
communications. 2016;11(12):1859– 60.
-
13. Tavadyan LA, Minasyan SH. Synergistic and antagonistic coantioxidant effects of flavonoids with trolox or ascorbic acid in a binary mixture. J Chem Sci. 2019 May 9;131(5):40.
-
14. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination
studies. Pharmacol Rev. 2006 Sep;58(3):621–81.
-
15. Susanto SW, Ranggaini MD. Aktivitas antioksidan ekstrak etanol rimpang curcuma xanthorrhiza Roxb. dan asam askorbat (Dengan metode DPPH, FRAP, dan H2O2). Jurnal Kedokteran Gigi Terpadu. 2022;4(1):83–33.
-
16. Setiawan PYB, Kertia N, Nurrochmad A, Wahyuono S. Synergistic antiinflammatory effects of Curcuma xanthorrhiza rhizomes and Physalis angulata herb extract on
lipopolysaccharide-stimulated RAW 264.7 cells. Journal of Applied Pharmaceutical Science. 2022 Jul 5;12(7):88–98.
-
17. Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. Lwt - Food Science and Technology. 2004 Nov 1;37:717– 21.
-
18. Purwakusumah ED, Royani L, Rafi M. Evaluasi Aktivitas Antioksidan dan Perubahan Metabolit Sekunder Mayor Temulawak (Curcuma xanthorriza) Pada Umur Rimpang Yang Berbeda. Jurnal Jamu Indonesia. 2016;1(1):10–7.
-
19. Widyastuti I, Luthfah HZ, Hartono YI, Islamadina R, Can AT, Rohman A. Antioxidant Activity of Temulawak (Curcuma xanthorrhiza Roxb.) and its Classification with Chemometrics. Indonesian J ChemomPharm Anal. 2020 Jul 19;29.
-
20. Alafiatayo AA, Syahida A, Mahmood M. Total anti-oxidant capacity, flavonoid, phenolic acid and polyphenol content in ten selected species of Zingiberaceae rhizomes. Afr J Tradit Complement Altern Med. 2014;11(3):7–13.
-
21. Devitria R, Sepriyani H, Sari S. Uji Aktivitas Antioksidan Ekstrak Metanol Daun Ciplukan Menggunakan Metode 2,2-Diphenyl 1-Picrilhidrazyl (DPPH).
Cjf JrpSA
Jurnal Penelitian Farmasi Indonesia. 2020;9(1):31–16.
-
22. Sari GNF. Aktivitas Antioksidan Ekstrak Dan Fraksi Herba Ciplukan (Physalis Angulata) Terhadap DPPH (1,1-Difenil-2-Pikrilhidrazil).
Prosiding Seminar Nasional Unimus. 2018;1:98–103.
-
23. Wang S, Zhu F. Dietary antioxidant synergy in chemical and biological systems. Crit Rev Food Sci Nutr. 2017 Jul 24;57(11):2343–57.
-
24. Putra IMWA, Fakhrudin N, Kusumawati IGAW, Nurrochmad A, Wahyuono S. Antioxidant properties of extract combination of Coccinia grandis and Blumea balsamifera: An in vitro synergistic effect. J Herbmed Pharmacol. 2021 Nov 29;11(1):55–62.
-
25. Setiawan PYB, Nyoman K, Arief N, Subagus W. Curcumin in combination: Review of synergistic effects and mechanisms in the treatment of inflammation. Journal of Applied Pharmaceutical Science. 2020 Feb
5;11(2):1–11.
92
Discussion and feedback