THE EFFECT OF VARIOUS LOCATION AND SOLVENT TO SAMBILOTO (Andrographis paniculata (Burm.f.) Nees) PHYTOCHEMICAL PROFILES
on

Journal Pharmaceutical Science and Application Volume 5, Issue 2, Page 77-84, December 2023 E-ISSN: 2301-7708
THE EFFECT OF VARIOUS LOCATION AND SOLVENT TO SAMBILOTO (Andrographis paniculata (Burm.f.) Nees) PHYTOCHEMICAL PROFILES
I Putu Priyasana1, Agung Endro Nugroho2, Soni Siswanto2, Dyaningtyas Dewi Pamungkas Putri2, Yosi Bayu Murti3*
-
1Master in Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Gadjah Mada, Jl. Sekip Utara, Sleman, Yogyakarta 55281, Indonesia.
-
2Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
-
3Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Gadjah Mada, Yogyakarta 55281, Indonesia
Corresponding author email: yosibayu.murti@ugm.ac.id
ABSTRACT
Background: Sambiloto (Andrographis paniculata (Burm.f.) Nees) is one of the traditional medicines that contain multiple phytochemical compounds. Every location has its characteristic that affects the metabolic process of phytochemical compounds. Apart from that, the extraction solvent also plays an essential role in attracting compounds in sambiloto. The solvent is related to the polarity and can affect the phytochemical profile. Objective: This research aims to determine the effect of the various location and extraction solvent of sambiloto on its phytochemical profile. Methods: Sambiloto from Denpasar (Dps), Sukoharjo (Skj), and Sleman (Slm) was extracted (1:10 w/v) using ultrasonic-assisted extraction in methanol 100% (MeOH100) and methanol 50% (MeOH50). In water solvents, extraction is carried out using the infusion method. Fingerprint analysis using HPTLC-Densitometry. The data obtained was analyzed further using principal component analysis (PCA). Results: Fingerprint analysis obtained peak data that varied in each extraction solvent. There are four major peaks in the chromatogram profile of each solvent. The peak chromatogram for each solvent also shows differences at each location. PCA analysis shows that the phytochemical content of sambiloto extract is divided into three main clusters. The distribution of the clusters is based on variations in the extraction solvent. Variations in location for each sambiloto also influence its phytochemical profile. However, these variations are still in the same quadrant for each solvent. Conclusion: The solvent determines more variations in the phytochemical profile of sambiloto extract than the various location.
Keywords: Sambiloto; Solvent; Location; Phytochemical; Principal Component Analysis
INTRODUCTION
Sambiloto (Andrographis paniculata (Burm.f.) Nees) is a plant often found in Indonesia. Sambiloto is generally used as a traditional medicine to treat various diseases[1,2]. Andrographolide is the main compound in sambiloto. Apart from that, there are also various phytochemical compounds, such as flavonoids, phytosterols, terpenoids, and xanthone[2]. An important factor that can influence the phytochemical profile of a plant is the location where it grows. The plant's location, which includes parameters such as climate, soil type, altitudinal, and geography, can contribute significantly to the type and concentration of chemical compounds produced by the plant. Therefore, understanding the influence of various location on plant phytochemical profiles has important implications in various fields, including pharmacology[3].
The environmental conditions in which plants grow vary widely worldwide, including different climates, soil nutrients, altitude differences, and other geographic factors. All these trigger changes in plant's physiological and biochemical responses to survive in changing environmental conditions. As a result, plants can produce different chemical compounds in various environmental conditions. Royani (2014) have found that sambiloto in different locations can significantly differ in phytochemical profiles, which one andrographolide[3,4].
In addition, type of solvent also significantly impacts the phytochemical profile of the extraction results. Solvents play a role in dissolving certain compounds from plant materials; each solvent has a different affinity for these compounds. Therefore, research on the effect of extraction solvents on the phytochemical profile of sambiloto is very important to understand the compounds it can extract.
Several factors that must be considered in solvent selection include solvent polarity, extraction temperature, and contact time. Various chemical compounds with different biological activities can be extracted using different solvents, thereby affecting the health benefits or efficacy of sambiloto[5,6].
Various methods can be used to analyze the phytochemical profile of sambiloto. TLC-Densitometry is a method can be used in phytochemical fingerprint analysis. The advantage of this method is can analyze samples simultaneously and provides reproducible results. The results obtained can be further analyzed using chemometrics. In chemometrics, one type of data processing technique is principal component analysis (PCA). Smaller dimensions from the feature space (independent variables) are obtained using PCA by reducing big data dimensions from the data space (observed variables)[7].
Chemometrics has been widely used for various chemical data analysis purposes. Sambiloto from different habitats will have varying contents, resulting in varying data, so chemometric methods are needed to process the data. The differences in independent variables can be grouped into clusters using PCA[8].This research focuses on how solvent variations in the extraction process and various location influence the phytochemical profiles of sambiloto in Indonesia. The phytochemical profile obtained can be used as quality control for data in scientific development.
METHODS
-
1. Materials
The aerial part of sambiloto (Andrographis paniculata (Burm. f.) Nees) was collected from 3 different locations in Indonesia. The location including Denpasar (Bali), Sukoharjo (Central Java), and Sleman (Yogyakarta). Aqua distillate and methanol were used as extraction solvents.
Silica gel HPTLC 60 F254, n-hexane, and ethyl acetate were used for phytochemical fingerprint analysis. All solvents used are pro analytical grade.
The samples are subjected to a dry sorting process, ensuring no impurities. The collected samples were then dried using an oven at 50ºC for 24 hours. Next, the dried samples were powdered with stamper and mortar. The powder obtained is then subjected to an extraction process.
Sambiloto powder from each region was dissolved separately in 100% methanol, 50% methanol and hot water (90ºC). The ratio of ingredients to solvent is 1:10 (w/v). Extraction in 100% and 50% methanol solvent was performed in an ultrasonic bath with a frequency of 20 kHz for 15 minutes. Meanwhile, the sample is heated to a temperature of 90ºC for 15 minutes in the water solvent. Replication was carried out by three individuals at each location so that the total extract to be tested was 27.
-
4. Phytochemical Fingerprint Analysis
Fingerprinting analysis of the sambiloto extract was carried out using the HPTLC-Densitometry method. The mobile phase is hexane–ethyl acetate (1:4 v/v). The volume of extract is 2 μL, width 6 mm with a distance between bands of 10 mm. Scanning were carried out using a TLC scanner 3 equipped with WinCATS 3.4 software (Camag) at a wavelength of 232 nm. Rf and peak area of the chromatogram will be analyzed further using PCA. Minitab 21.4.1 was used to analyze the PCA.
RESULTS
Fingerprint analysis obtained peak data that varied in each extraction solvent (Figure 1.). There were seven peaks in the sambiloto extract with MeOH50 and water solvents. Meanwhile, the MeOH100 extract has nine peaks. The peak chromatogram
results for each solvent also show differences at each location. This difference is based on the relative levels of each peak produced. The MeOH100 extract gave four major spots, which appeared at Rf 0.69, 0.77, 0.85, and 0.96. Four major spots were detected in the MeOH50 extract at Rf 0.66, 0.75, 0.84, and 0.98. Four major spots have been detected in water extract, at Rf 0.57, 0.67, 0.77, and 0.94. Each peak has a different relative concentration at each various location and extraction solvent. PCA analysis was used further to see the pattern of variations in the presence of compounds and relative levels at the growth location and extraction solvent.
PCA obtained shows that the phytochemical content of sambiloto extract is divided into three main clusters (Figure 2.). The distribution of the clusters is based on variations in the extraction solvent. MeOH100 extract is in the quadrant with negative PC1 and PC2. The MeOH50 extract is in the quadrant with positive PC1 and negative PC2. The water extract shows a negative distribution of PC1 and PC2. Even though it is in the negative quadrant, the effect of water extract is more towards positive PC1 and PC2. Variations in location for each sambiloto also influence its phytochemical profile. However, these variations are still in the same quadrant for each solvent.
DISCUSSION
Sambiloto contains various compounds such as phytosterols, flavonoids, tannins, and terpenoids. The existence of all these compounds can be influenced by two factors, namely intrinsic and extrinsic. Intrinsic factors are influenced by genetics and the source of plant seeds. Extrinsic factors are influenced by geographic location, climate, and environment. In this study, three areas were selected with different characteristics.
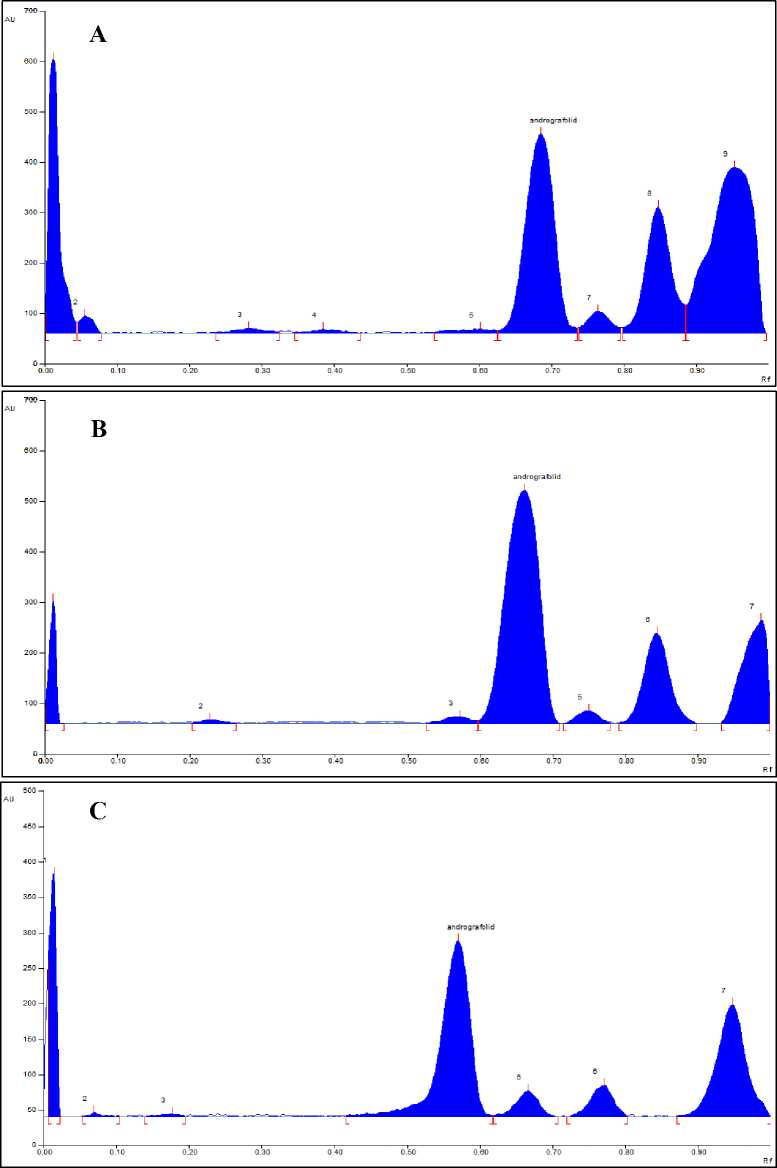
Figure 1. Peak Chromatogram from Sambiloto Extract. (A) Denpasar-Methanol 100%; (B) Sukoharjo-Methanol 50%; (C) Sleman-Water.
Sambiloto harvested at the Denpasar location grows under the shade of other plants and near the beach area. At the Sukoharjo location, Sambiloto grows scattered without shadow in hilly areas. Sambiloto in the Sleman location grows in the shade, close to a large river. The fingerprint pattern at various locations shows four major peaks, including andrographolide obtained at each location (Figure 1.). However, the differences are more visible in other small peaks at each location. Furthermore, differences were also shown in the relative levels of each peak in various extraction solvents. Research by Dong et al. (2009) showed that the
fingerprints of sambiloto extract at each location showed the same dominant peak, but other non-dominant peaks were different at each location. Although fingerprints from different locations can be distinguished visually, the process is subjective and not quantitative. Additionally, small differences between very similar chromatograms may be missed [9]. Research by Sharma et al. (2011) showed varying data on phytochemical compounds for 15 different sambiloto genotypes. Apart from that, the levels of andrographolide as a marker compound produce different amounts[10].
Score Plot Phytochemical Profiles from Sambiloto Extract
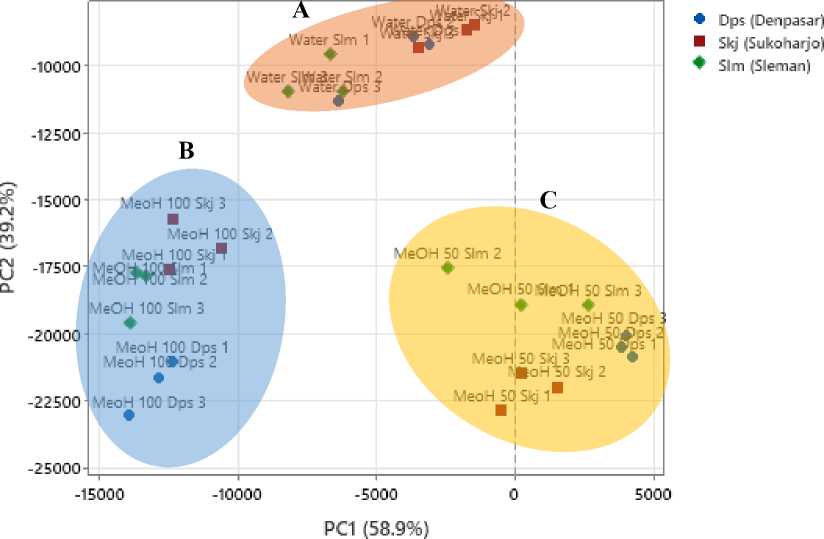
Figure 2. Score Plot Phytochemical Profiles from Sambiloto Extract. (A) MeOH 100 (Methanol 100%) Extract Cluster; (B) MeOH 50 (Methanol 50%) Extract Cluster; (C) Water Extract Cluster; Dps (Denpasar); Skj (Sukoharjo); Slm (Sleman)
Selecting the extraction solvent also has a critical role in the compound content of an extract. The extraction solvent is related to the polarity of the solvent concerning which the compound can be dissolved. Each compound has different solubility characteristics in each solvent[11]. Methanol is a semi-polar solvent which has a high polarity range. Methanol can attract polar to semi-polar compounds. So that more compound components are drawn compared to using polar solvents. Water solvents are limited in attracting only polar compounds[12]. Research by Srivastava (2004) analyzed solvent variations in sambiloto extract using the HPTLC method. The results were variations in the phytochemical profile of extracts with n-hexane, methanol, chloroform, and water solvents. The phytochemical profile of the n-hexane solvent showed that there were no andrographolide compounds. Meanwhile, the highest andrographolide peak was obtained in methanol extract[13]. Variations concentration from methanol and ethanol also influence the phytochemical compound of the sambiloto[5,11]. Research by Rao and Rathod (2015) shows that methanol solvent produces the highest levels of andrographolide[14]. Similar results were also shown by research by Rafi et al. (2020), who found that water extract produced the lowest andrographolide levels. Research by Wattananat et al. (2016) showed that 50% methanol extract of sambiloto produced the highest levels of andrographolide using ultrasonic extraction[5,15].
Principal component analysis is used in this analysis to process Rf and AUC in each extract into simple data. PCA reduce the dimensions of a data set and interpret the data while preserving the most essential information from the data set. The data is obtained by generating principal components (PCs) as an orthogonal linear
transformation that considers the variance of the various variables in the data. PC1 describes the highest variance in the data. This means PC1 will explain as much variation as possible in the original data. PC2 is the second main component with high variance, but not as much as PC1. However, PC2 is orthogonal or uncorrelated with PC1. In other words, PC2 is a linear combination of the original variables that differs from PC1 and explains additional variations in the data that PC1 cannot explain. Excellent data visualization may be obtained when the combined proportion of PC1 and PC2 is more than 70%. The total percentage data for PC1 and PC2 acquired in this analysis was 98.1%. There are three main clusters, which are divided based on differences in extraction solvents. The three clusters show different characteristics based on their contribution to PCs. MeOH100 extract showed a negative contribution to two PCs. This indicates that the extract has a strong impact on PC1 and PC2, but its contribution is negative. MeOH100 extract does not contribute to the variation in the data explained by the two PCs. In contrast, PC1 and PC2 are more influenced by other samples in this data set[5,16].
The MeOH50 extract, it is in the quadrant with PC1 positive and PC2 negative. This indicates that the MeOH50 extract significantly contributes to PC1 in the data variation. Data on MeOH50 extract has a strong impact on PC1 variations. However, the MeOH50 extract did not significantly contribute to variations in PC2. The water extract is the same as the MeOH100 extract, but the effect on the water extract is more positive towards PC1 and PC2. This extract indirectly contributed little to the variations in PC1 and PC2. Variations in location for each sambiloto show different positions but are still in the same quadrant for variations in solvent.
These results align with research by Zhao et al. (2014), which showed that fingerprint patterns of sambiloto extract from several locations were grouped in the same quadrant. This shows that variations in location did not show significant differences in phytochemical profiles in the sambiloto extract[16]. The effect of solvent variations shows more significant difference compared to the effect of differences in location. Research by Rafi et al. (2020) showed that phytochemical fingerprint analysis of pure ethanol, 70%, 50%, 30%, and water solvents showed almost the same pattern. Several peaks are the same, only differ in relative concentration. Grouping results also occurred based on variations in extraction solvents[5]. This research shows that various location in Indonesia show similar phytochemical profile sambiloto extract, especially andrographolide. Sambiloto can be further processed at any location. However, to obtain the best andrographolide peak, 50% methanol solvent can be used with the ultrasonic extraction method.
CONCLUSION
There is an influence of variations in extraction solvent on the phytochemical profile of sambiloto extract. Varying the various location has a less strong influence when compared to varying the extraction solvent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
This research was supported by the National Research & Innovation Agency -Endowment Fund for Education (BRIN -LPDP).
REFERENCES
-
1. Jiang M, Sheng F, Zhang Z, Ma X, Gao T, Fu C, et al. Andrographis paniculata (Burm.f.) Nees and Its Major Constituent Andrographolide As Potential Antiviral Agents. Journal of Ethnopharmacology. 2021;272:1– 16.
-
2. Kumar S, Singh B, Bajpai V. Andrographis paniculata (Burm. f.) Nees: Traditional Uses,
Phytochemistry, Pharmacological Properties and Quality
Control/Quality Assurance. Journal of Ethnopharmacology. 2021;275:1–30.
-
3. Royani JI, Hardianto D, Wahyuni S.
Analisa Kandungan Andrographolide pada Tanaman Sambiloto
(Andrographis paniculata) dari 12 Lokasi di Pulau Jawa. J Bioteknol Biosains Indones. 2014 Dec 26;1(1):15–20.
-
4. Sareer O, Ahmad S, Umar S. Andrographis paniculata : A Critical Appraisal of Extraction, Isolation and Quantification of Andrographolide and Other Active Constituents. Natural Product Research. 2014 Dec 2;28(23):2081–101.
-
5. Rafi M, Devi AF, Syafitri UD, Heryanto R, Suparto IH, Amran MB, et al. Classification of Andrographis paniculata Extracts by Solvent Extraction Using HPLC Fingerprint and Chemometric Analysis. BMC Research Notes. 2020;13(1):1–6.
-
6. Song YX, Liu SP, Jin Z, Qin JF, Jiang ZY. Qualitative and Quantitative
Analysis of Andrographis paniculata by Rapid Resolution Liquid
Chromatography/Time-of-Flight Mass Spectrometry. Molecules. 2013 Sep 30;18(10):12192–207.
-
7. Miller JN, Miller JC. Statistics and Chemometrics for Analytical
Chemistry. 5th ed. Harlow, England ; New York: Pearson Prentice Hall; 2005.
-
8. Islamadina R, Chan A, Rohman A. Chemometrics Application for Grouping and Determinating Volatile Compound which related to Antioxidant Activity of Turmeric Essential Oil (Curcuma longa).
2020;8(2):225–39.
-
9. Dong HJ, Zhang ZJ, Yu J, Liu Y, Xu FG. Chemical Fingerprinting of Andrographis paniculata (Burm. f.) Nees by HPLC and Hierarchical Clustering Analysis. Journal of Chromatographic Science. 2009 Nov 1;47(10):931–5.
-
10. Sharma SN, Jha Z, Sharma DK. Chemometrics Evaluation of the
Herbal Drug Andrographis
paniculata. Natural Product
Communications. 2011
Dec;6(12):1929–32.
-
11. Kumoro AC, Hasan M, Singh H. Effects of solvent properties on the Soxhlet extraction of diterpenoid lactones from Andrographis
paniculata leaves. ScienceAsia. 2009;35(3):306–9.
-
12. Snyder LR, Kirkland JJ, Dolan JW. Introduction to Modern Liquid Chromatography. Third Edition. New Jersey, USA: John Wiley & Sons, Inc.; 2010.
-
13. Srivastava A, Misra H, Verma RK, Gupta MM. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and Densitometry. Phytochem Anal. 2004
Sep;15(5):280–5.
-
14. Rao PR, Rathod VK. Mapping Study of An Ultrasonic Bath for The Extraction of Andrographolide From Andrographis paniculata Using
Ultrasound. Industrial Crops and Products. 2015;66:312–8.
-
15. Wattananat T, Duangteraprecha S, Jamtaweekul J, Sukphan P. Alternative to the Thai Herbal Pharmacopoeia Method for Quality Control of Andrographis Capsules. Journal of Thai Traditional and Alternative Medicine. 2016;14:314– 24.
-
16. Noviana E, Indrayanto G, Rohman A. Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Front Pharmacol. 2022 Jun 2;13:1–21.
-
17. Zhao Y, Wu KC, Liao CR, Ho YL, Chang YS. Chemical Compositions, Chromatographic Fingerprints and Antioxidant Activities of
Andrographis Herba. Molecules. 2014 Nov 10;19(11):18332–50.
84
Discussion and feedback