IN SILICO TOXICITY OF ANTHOCYANIN COMPOUNDS (CYANIDIN AND PEONIDIN)
on

Journal Pharmaceutical Science and Application Volume 5, Issue 1, Page 53-58, June 2023 E-ISSN: 2301-7708
IN SILICO TOXICITY OF ANTHOCYANIN COMPOUNDS (CYANIDIN AND PEONIDIN)
Ni Made Pitri Susanti1*, I Nyoman Triadhi Wisesa1, Chenme Juwianti1
1Study Program of Pharmacy, Faculty of Mathematics and Natural Sciences, Universitas Udayana, Badung-Indonesia
Corresponding author email: dekpitsusanti@unud.ac.id
ABSTRACT
Backgrounds: Cyanidin and peonidin, anthocyanin compounds, have many in silico, in vitro, and in vivo activities, including antioxidant, anticancer, antihyperlipidemic, and antidiabetic. Toxicity testing is carried out to determine the potential hazard that may be produced by the test compound. Objective: This study was aimed to determine the in silico toxicity of anthocyanins (cyanidin and peonidin) using Toxtree v3.1.0 software. Methods: In silico toxicity testing was carried out using 2D structures of cyanidin and peonidin with Cramer rules, Verhaar scheme, Benigni/Bossa rulebase, Kroes TTC decision tree, Eye Irritation/Corrosion and Skin Irritation/Corrosion parameters. Data analysis on the results of the tested toxicity parameters was carried out descriptively. Results: The results showed that the two compounds have the same category for the toxicity parameters of Cramer Rules (class III), Kroes TTC Decision Tree (substance would not be expected to be safety concern), Benigni/Bossa Rulebase (Negatif for genotoxic and nongenotoxic carcinogenicity), Eye Irritation/Corrosion and Skin Irritation/Corrosion (not irritating or corrosive). Different results are shown in the parameters of the Verhaar Scheme, where cyanidin is included in class 5 (cannot be classified based on this parameter), while peonidin is included in class 1 (narcosis or basic toxicity). Conclusion: Based on in silico toxicity, cyanidin and peonidin have a chemical structure that has the potential for toxicity, but these compounds are neither potentially genotoxic nor non-genotoxic carcinogenicity, and are not potentially toxic to the skin and eyes. The toxicity mechanism of cyanidin cannot be classified based on the test parameters while peonidin is narcosis or basic toxicity.
Keywords: Anthocyanin; Cyanidin; Peonidin; Toxicity; In Silico
INTRODUCTION
Anthocyanins are a class of flavonoids found in plants with the characteristic of giving bright colors such as orange, red and blue. There are six types of anthocyanins commonly found in higher plants, namely: cyanidin, pelargonidin, petunidin, malvidin, peonidin, and delvinidin[1]. Many plants contain anthocyanins, one of which is the purple sweet potato (Ipomoea batatas L.). Anthocyanin in purple sweet potato is known to have activity as an antioxidant,
anti-inflammatory, anticarcinogenic, antiulcer, hepatoprotective, and hypouricemia[2-7]. In purple sweet potato root, the highest anthocyanin content is cyanidin and peonidin [2]. In silico, cyanidin and peonidin also have the potential to inhibit porcine pancreatic α-amylase as anti-diabetic type 2, inhibit superoxide dismutase as an antioxidant, and HMG-CoA reductase inhibitors as antihyperlipidemia[8-10].
53
In drug development, besides activity, toxicity also needs to be considered. Toxicity tests determine the potential hazard of compounds that may be produced[11]. Initial toxicity testing can be carried out in silico without using experimental animals, so that toxicity tests can be carried out more quickly and efficiently in predicting the toxicity of a compound[12]. In silico method was developed to predict the toxicity of a compound based on the relationship between its physicochemical properties and biological activity including its toxicity effects, using certain algorithms and database models[13]. In this study, toxicity test of the anthocyanin compounds, cyanidin and peonidin, was carried out using Toxtree v3.1.0. to predict toxicity levels, mutagenesis and carcinogenesis, as well as irritation or corrosion of skin and eyes in silico[14-15].
METHODS
The 2D structures of cyanidin and peonidin were downloaded from https://pubchem.ncbi.nlm.nih.gov. with SDF file format (*.sdf) (Figure 1). Toxicity prediction was performed using Toxtree v3.1.0 software. Tests were carried out using six parameters, namely Cramer Rules, Verhaar Scheme, Benigni/Bossa Rulebase, Kroes TTC Decision Tree, Eye Irritation/Corrosion, and Skin Irritation/Corrosion. The data obtained were analyzed descriptively.
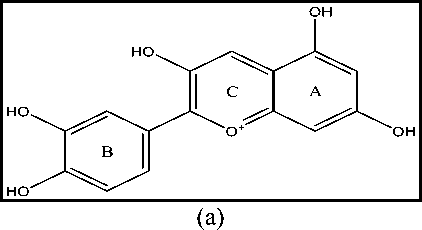
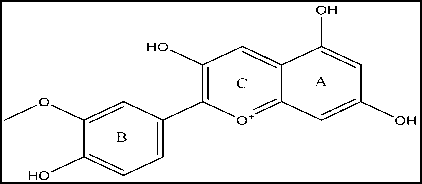
(b)
Figure 1. 2D Structure of Cyanidin (a) and Peonidin (b)
RESULTS
The results of the cyanidin and peonidin toxicity tests for the 6 test parameters are shown in Table 1. Cyanidin and peonidin showed the same category for the toxicity parameters of Cramer Rules, Benigni/Bossa Rulebase, Kroes TTC Decision Tree, Eye Irritation/Corrosion, and Skin Irritation/Corrosion. Different results are shown in the parameters of the Verhaar Scheme, where cyanidin is included in class 5 (cannot be classified based on this parameter), while peonidin is included in class 1 (narcosis or basic toxicity).
DISCUSSION
Toxtree has many test parameters with different toxicity test results[16]. The Cramer rules, Verhaar scheme, and Benigni/Bossa rulebase toxicity parameters only require the 2D structure of the test compound, while the Kroes TTC decision tree, Eye Irritation/Corrosion and Skin Irritation/Corrosion parameters require additional data, estimated daily intake (μg/day) and melting point.
Cramer rule parameters are used to classify and rank compounds for oral toxicity. This parameter classifies compounds into three classes, namely: Class I, Class II, and Class III. Questions about this parameter are based on knowledge of toxicity and metabolism in mammals[17]. Cyanidin and peonidin are classified into class III, a group of compounds with a chemical structure that
54
allows for significant toxicity or toxicity[15]. This is based on the structure of cyanidin and peonidin which have heterocyclic, heteroaromatic, substituted rings, and more than one aromatic ring. Based on Figure 1, the heterocyclic and heteroaromatic structures of cyanidin and peonidin are ring C which is an oxidation form of the pyran ring of flavonoids, while the aromatic rings are rings A and B[18]. Compounds with heterocyclic or heteroaromatic rings have the intermediate metabolism product of arene oxide, which is reactive compound, react with nucleophiles, and react spontaneously to DNA causing carcinogenesis and mutagenesis. The benzene ring with -OH substituent is metabolized through oxidative reaction to become reactive electrophile compound that can cause oxidative stress. Increasing the number of aromatic rings will increase the irreversible inhibitory effect of cytochrome P450 thereby increasing the risk of toxicity [19-21].
The Kroes TTC decision tree is based on Cramer's rules to estimate the exposure threshold based on dose-response for compounds with a carcinogenic risk[14,22]. This parameter requires daily intake data. The daily intake of cyanidin and peonidin compounds is unknown, therefore the daily intake data is used from the results of the Cramer rule parameter test, which is 90 μg/day[23]. The test results show that cyanidin and peonidin are included in compounds with substances that would not be expected to be a safety concern. Cyanidin and peonidin do not have structures indicating potential genotoxic carcinogenicity based on the 39 structural alerts used in this parameter [23,24].
The Verhaar scheme is used to predict the mechanism of toxicity in acute toxicity[25]. This parameter can be divided into five classes, namely: Class 1, 2, 3, 4, and 5. The test results show that cyanidin belongs to class 5, which is a group of compounds that cannot be classified based
on the parameters of the Verhaar scheme so that further testing is required[14].
|
Table 1. Toxicity Test Results of Cyanidin and Peonidin | ||
|
Test Parameter |
Compound | |
|
Cyanidin |
Peonidin | |
|
Cramer rules |
Class III (High) |
Class III (High) |
|
Kroes TTC decsion tree |
Substance would not be expected to be safety concern |
Substance would not be expected to be safety concern |
|
Verhaar scheme |
Class 5 (Not Possible to classify according to these rules) |
Class 1 (narcosis or baseline toxicity) |
|
Benigni/Bossa rulebase |
Negatif for genotoxic and nongenotoxi c carcinogeni city |
Negatif for genotoxic and nongenotoxic carcinogenicit y |
|
Skin Irritation/ Corrosion |
Non irritating or corrosive to skin (does not cause burns and severe burns) |
Non irritating or corrosive to skin (does not cause burns and severe burns) |
|
Eye Irritation/ Corrosion |
Non irritating or corrosive to eye (does not cause burns and severe burns) |
Non irritating or corrosive to eye (does not cause burns and severe burns) |
Peonidin belongs to class 1 which is a compound with basic toxicity. Compounds with basic toxicity have a toxicity mechanism called narcosis, which is a reversible suppression of physiological functions due to the hydrophobic bond of the compound to cell membranes and proteins. Compounds with this toxicity mechanism have toxic effects through disruption of membrane function[26]. Class 1 compounds are not
55
reactive and do not interact specifically with receptors on organisms[14].
Benigni/Bossa rulebase parameters are used to predict the carcinogenic and mutagenic potential of a compound based on the structural alerts model[14.24.27]. The mechanism of carcinogenicity consists of genotoxic and non-genotoxic. Based on the test results, cyanidin and peonidin are predicted not to cause genotoxic or nongenotoxic carcinogenicity. This result was obtained because the cyanidin and peonidin compounds did not contain any of the structural alerts present in this parameter.
Parameters Eye Irritation/Corrosion and Skin Irritation/Corrosion are used to assess the potential toxicity of the compounds to the skin and eyes. This parameter uses the physicochemical properties of the melting point. The test results show that cyanidin and peonidin are not irritating or corrosive (do not cause burns and severe burns). This is because both compounds have high melting points, above 200ºC[28]. The melting point is related to the permeability of compounds on the skin as well as their solubility and ability to penetrate the skin[29].
Compounds with melting points below 100ºC are more easily absorbed through the skin, so the possibility of causing corrosion or irritation is higher[30,31].
CONCLUSION
Based on in silico toxicity, cyanidin and peonidin compounds have a chemical structure that allows for toxicity with a cyanidin toxicity mechanism that cannot be classified based on test parameters, while peonidin is marcosis or basic toxicity. These two compounds do not have the potential to cause carcinogenicity either genotoxic or nongenotoxic, and are not potentially toxic to the skin or eyes.
CONFLICT OF INTEREST
There is no conflict of interest in the preparation of this article.
ACKNOWLEDGEMENT
The authors would like to thank all parts that support this research.
REFERENCES
-
1. Goulas V, Vicente AR, Manganaris GA. Structural Diversity of Anthocyanins in Fruits. In: Motohashi N, editor. Anthocyanins: Structure, Biosynthesis and Health Benefits. New York: Nova Science Publishers, Inc., 2012. pp. 225-50.
-
2. Jiao Y, Jiang Y, Zhai W, Yang Z. Studies on Antioxidant Capacity of Anthocyanin Extract from Purple Sweet Potato (Ipomoea batatas L.). African J of Biotech. 2012; 11(28): 7046-54.
-
3. Kang H, Kwak YG, Koppula S. Protective Effect on Purple Sweet Potato (Ipomoea batatas Linn,
Convolvulaceae) on
Neuroinflammatory Responses in Lipopolysaccharide-Stimulated Microglial Cells. Trop J of Pharm Res. 2014; 13(8): 1257-63.
-
4. Margaret TM, Krishna P, Revathi B, Tony DE, Kumar MS, Babu AN. 2013. Asessment of In Vitro Anti Inflamatory Activity of Aqueous Extract of Ipomoea batatas Tubers. Asian J of Res in Biol and Pharm Sci. 2013; 1(1): 47-53.
-
5. Montilla EC, Hillebrand S, Winterhalter P. Antocyanins in Purple Sweet Potato (Ipomoea batatas L.) Varieties. Fruits, Vegetable and Cereal Science and Biotechnology. 2011; 5(2): 19-24.
-
6. Shipp J, Abdel-Aal ESM, Food Applications and Physiological Effects of Anthocyanins as Functional Food Ingredients. The Open Food Science Journal. 2010; 4: 7-22.
-
7. Zhang ZC, Su GH, Luo CL, Pang YL, Wang L, Li X et al. Effects of Anthocyanins from Purple Sweet Potato (Ipomoea batatas L. cultivar 56
Eshu No. 8) on The Serum Uric Acid Level and Xanthin Oxidase Activity in Hyperuricemic Mice. Food Funct. 2015; 6(9): 3045-55.
-
8. Laksmiani NPL, Paramita NLPV,
Wirasuta IMAG, In Vitro and In Silico Antioxidant Activity of Purified Fractions from Purple Sweet Potato Ethanolic Extract. Int J of Pharm and Pharm Sci. 2016; 8(8): 177-81.
-
9. Susanti NMP, Warditiani NK,
Laksmiani NPL, Dewi NKAS, Oka M, Heltyani WE, Wicaksana GPAP, Wirasuta IMAG. HMG-CoA Reductase Inhibitor Activity of Anthocyanin from Purple Sweet Potato (Ipomoea batatas L.). In: Proceedings of The International Conference on Biosciences. 2016 Jul 26-27; Denpasar, Indonesia, 2016. p. 89-94.
-
10. Sui X, Zhang YA, Zhou W. In Vitro and In Silico Studies of The Inhibition Activity of Anthocyanins Against Porcine Pancreatic α-Amylase. J of Funct Foods. 2015; 21: 50-57.
-
11. Arome D, Chinedu E. 2013. The Importance of Toxicity Testing. J of Pharm and BioSci. 2013; 4: 146-8.
-
12. Raunio H. In Silico Toxicology: Non Testing Methods. Front in Pharm. 2011; 2(33): 1-8.
-
13. Damayanti S, Permana J, Tjahjono DH. The Use of Computational Chemistry to Predict Toxicity of Antioxidants Food Additives and Its Metabolites as A Reference for Food Safety Regulation. Der Pharma Chemica. 2015; 7(9): 174-81.
-
14. Ideaconsult. Toxtree User Manual. Bulgaria: Ideaconsult Ltd; 2009 p. 166.
-
15. Patlewicz G, Jeliazkova N, Safford RJ, Worth AP, Aleksiev B. An Evaluation of The Implementation of The Cramer Classification Scheme in The Toxtree Software. SAR and
QSAR in Environmental Research. 2008; 19(5): 495-524.
-
16. Cronin MTD, Madden JC, Enoch SJ, Roberts DW. Chemical Toxicity Prediction Category Formation and Read-Across. UK: The Royal Society of Chemistry; 2013.
-
17. Lapenna S, Worth A. Analysis of the Cramer classification scheme for oral systemic toxicity - implications for its implementation in Toxtree. EUR 24898 EN. Luxembourg
(Luxembourg): Publications Office of the European Union; 2011. JRC66022
-
18. Welch CR, Wu Q, Simon JE. Recent Advances in Anthocyanin Analysis and Characterization. Curr Anal Chem. 2008; 4(2): 75-101.
-
19. Obach RS. Functional Group Biotransformations. In: Lee PW, Aizawa H, Gan LL, Prakash C, Zhong D, editors. Handbook of Methabolic Pathways of Xenobiotics. 1st ed. USA: John Willey & Sons, Ltd. 2014. pp. 1-41.
-
20. Ritchie TJ, Macdonald SJF, The Impact of Aromatic Ring Count on Compound Developability – Are too Many Aromatic Rings A Liability in Drug Design?. Drug Discovery Today. 2009; 14(21): 1011-20.
-
21. Zhou S, Chan E, Li X, Huang M. Clinical Outcomes and Management of Mechanism–Based Inhibition of Cytochrome P450 3A4. Therapeutics and Clinical Risk Management. 2005; 1(1): 3-13.
-
22. Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, et al. Structure-based Thresholds of Toxicological Concern (TTC): Guidance for Apllication to Substances Present at Low Levels in The Diet. Food and Chemical Tox. 2004; 42: 65-83.
-
23. Kroes R, Renwick AG, Feron V, Galli CL, Gibney M, Greim H, et al. Application of The Theshold of 57
Toxicological Concern (TTC) to The Safety Evaluation of Cosmetic Ingredients. Food and Chemical Tox. 2007; 45: 2533-2562.
-
24. Benigni R, Bossa C, Tcheremenskaia O. Nongenotoxic Carcinogenicity of Chemicals: Mechanism of Action and Early Recognition Through A New Set of Structural Alerts. Chem Rev. Vol. 2013; 113(5): 2940-57.
-
25. Verhaar HJM, van Leeuwen CJ, Hermens JLM. Classifying
Environmental Pollutants. 1:
Structure-Activity Relationships for Prediction of Aquatic Toxicity. Chemosphere. 1992; 25(4): 471-91.
-
26. He J, Li JJ, Wen Y, Tai HW, Yu Y, Qin WC, et al. Investigation on Modes of Toxic Action to Rats Based on Aliphatic and Aromatic Compounds and Comparison with Fish Toxicity Based on Exposure Routes. Chemosphere. 2015; 128:
111-117.
-
27. Benigni R, Bossa C, Jeliazkova N, Netzeva T, Worth A. The Benigni/Bossa Rulebase for
Mutagenicity and Carcinogenicity-A Module of Toxtree. Italy: European Communities. 2008. p. 1-70.
-
28. Gerner I, Schlegel K, Walker JD, Hulzebos E. Use of Physicochemical Property Limits to Develop Rules for Identifying Chemical Substances with No Skin Irritation or Corrosion Potential. QSAR & Com Sci. 2004; 23: 726-733.
-
29. Zapor L. Toxicity of Some Phenolic Derivatives-In Vitro Studies. International Journal of
Occupational Safety and
Ergonomics. 2004; 10(4): 319-31.
-
30. Ates G, Steinmetz FP, Doktorova TY, Madden JC, Rogiers V. Linking Existing In Vitro Dermal Absorption Data to Physicochemical Properties: Contribution to The Design of A Weight-of-Evidence Approach for
The Safety Evaluation of Cosmetic Ingredients with Low Dermal Bioavailability. Reg Tox and Phar. 2016; 76: 74-78.
-
31. Hulzebos EM, Maslankiewicz L, Walker JD. Verification of Literature-Derived SARs for Skin Irritation and Corrosion. QSAR & Combinatorial Science. 2003; 22:
351-63.
58
Discussion and feedback