PRODUCTION OF HUPERZINE A BY FUNGAL ENDOPHYTES ASSOCIATED WITH HUPERZIACEAE PLANTS
on

Journal Pharmaceutical Science and Application Volume 5, Issue 1, Page 45-52, June 2023 E-ISSN: 2301-7708
PRODUCTION OF HUPERZINE A BY FUNGAL ENDOPHYTES
ASSOCIATED WITH HUPERZIACEAE PLANTS
Ni Wayan Prasanthi Swarna Putri1, Ni Putu Ariantari1*
1Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Udayana University, Badung 80361, Bali, Indonesia.
Corresponding author email: putu_ariantari@unud.ac.id
ABSTRACT
Background: Endophytic fungi are known as a producer of a myriad of bioactive natural products, including those originally produced by the host plants. Huperzine A (Hup A), a lycopodium alkaloid, which was reported as an active acetylcholinesterase inhibitor (AChEI) agent used for the treatment of Alzheimer’s disease, was originally isolated from medicinal Chinese herbs Huperzia serrata. Moreover, various species of endophytic fungi associated with H. serrata and other species within Huperziaceae plants were found capable of producing Hup A, suggesting these microorganisms as promising sources of Hup A. Objective: This review aimed to summarize the evidence of Hup A produced by various endophytic fungi as part of efforts to assess alternative producer of Hup A. Methods: Scientific articles on endophytic fungi producing Hup A published from 2000 until 2022 were screened through scientific databases. Results: Thirty-two endophytic fungal strains belonging to fifteen fungal genera were documented as capable of producing Hup A. These fungal endophytes were isolated from Huperziaceae plants, H. serrata, Phlegmariurus phlegmaria and Phlegmariurus taxifolius. Conclusion: We summarize herein the capability of endophytic fungal strains associated with Huperziaceae plants to produce Hup A, an active AChEI agent, which could be considered as an alternative producer of Hup A on a larger scale.
Keywords: Acetylcholinesterase inhibitor (AChEI); Alzheimer’s disease; Fungal endophytes; Huperziaceae; Huperzine A (Hup A).
INTRODUCTION
Fungal endophytes are known as microorganisms inhabiting the inner parts of the host plants and are capable of producing various pharmacologically active natural products.[1] Endophytes were first reported in 1904 [2], however, these microorganisms drew attention years later after the finding of paclitaxel, an anticancer agent initially isolated as plant metabolite, produced by the endophytic fungus Taxomyces andreanae, which inhabiting Taxus brevifolia.[3] Taxus
brevifolia is known as the initial source of paclitaxel. Since then, many studies have been carried out to explore the capability of endophytic fungi associated with medicinal plants to produce pharmaceutically valuable natural products, leading to the discovery of other anticancer camptothecin among others. This metabolite was produced by the endophytic fungus Entrophospora infrequens, isolated from stems of Nothapodytes foetida.[4] This finding
revealed the prospect of utilizing fungal endophytes as alternative producers of bioactive metabolites initially derived from plants for various pharmacological effects, including compounds with acetylcholinesterase inhibitory activity.
Natural products active as acetylcholinesterase inhibitor (AChEI) are regarded as one of important therapeutic targets for the treatment of Alzheimer's disease (AD).[5] World Health Organization (WHO) reported around 55 million people in the world live with dementia, and 60-70% of these dementia cases are dominated by AD.[6] However, therapeutic options available for AD treatment are still limited, of which donepezil, galantamine, rivastigmine, and tacrine are the most common pharmaceutical products used for AD therapy so far.[7] In addition to these four medicines, a natural product called Huperzine A (Hup A), has been used for AD therapy in China for years, however the production of Hup A evoked major challenge for further clinical application.
Hup A, an active AChEI agent was initially isolated from the Chinese herb Huperzia serrata and has been approved in China for the treatment of AD since the 1990s.[8] The high demand for Hup A for use as an Alzheimer's drug indirectly pressures researchers to obtain Hup A in large quantities without risking the existence of H. serrata in nature. Efforts to preserve H. serrata by cultivation are not possible, because H. serrata is difficult to cultivate under natural conditions.[9] Therefore, it is necessary to find other ways to increase Hup A production, such as by utilizing endophytic fungi isolated from Huperziaceae plants or specifically H. serrata. Given by the possibility that fungal endophytes could produce the same metabolites as those of afforded from the host plant, the scientific approaches by
using microorganisms i.e. endophytic fungi associated with H. serrata are considered as a promising strategy to produce Hup A without endangering H. serrata population in nature.
A previous review by Cao et al. 2021 reported nine strains of fungal endophytes producing Hup A particularly obtained from H. serrata, which indicated their benefit as promising Hup A producers.[10] As production of Hup A was not only limited to fungal endophytes associated with H. serrata, in this review, we described endophytic fungal strains associated with Huperziaceae family which capable of producing Hup A. All in all, 16 scientific articles on endophytic fungi isolated from host plants belonging to Huperziaceae family that can produce Hup A were included in this review.
METHODS
Scientific articles on endophytic fungi producing Hup A published from 2000 until 2022 were screened through scientific databases (PubMed, ScienceDirect, BioMed Central, and Google Scholar). Keywords used for the literature search included acetylcholinesterase inhibitor, Alzheimer's disease, endophytic fungi, huperzine A, and Huperziaceae. Only scientific articles reporting Hup A production from Huperziaceae-derived endophytic fungi were included in this review.
RESULTS
The family Huperziaceae consists of two main genera, i.e. Huperzia and Phlegmariurus. One of the most popular species from Huperziaceae is Huperzia serrata.[11] Hup A (Figure 1) is produced by H. serrata and other species within Huperziaceae family which are closely related to H. serrata.[12] Hup A content in
Table 1 (Cont.)
wild H. serrata is relatively low. Moreover, H. serrata growth was very slow with a long-life cycle, which takes 15-20 years for this plant to mature.[9]
Figure 1: Chemical structure of huperzine A (Hup A)
In this review, thirty-two endophytic fungal strains from fifteen fungal genera isolated from host plants, H. serrata, Phlegmariurus phlegmaria, and Phlegmariurus taxifolius were reported capable of producing Hup A (Table 1). Among these genera, endophytic fungal strains belonging to Colletotrichum, Fusarium and Penicillium were frequently reported as producers of Hup A (Figure 1), as shown in Figure 2.
Table 1: Endophytic fungi belonging to the Huperziaceae family previously reported capable to produce Hup A
Huperzia serrata as host plant
|
Genera |
Strain |
Reference |
|
Acremonium |
A. implicatum LF30 |
[13] |
|
Alternaria |
A. brassicae AGF041 |
[14] |
|
Aspergillus |
A. flavus LF40 |
[13] |
|
Cladosporium |
C. cladosporioides LF70 |
[15] |
|
Colletotrichum |
C. |
[16] |
|
gloeosporioides ES026 |
[17] | |
|
C. gloeosporioides Cg01 |
[18] | |
|
C. boninense HS7-1 |
[19] | |
|
Fusarium |
Fusarium sp. Rsp5.2 |
[20] |
|
Genera |
Strain |
Reference |
|
F. verticillioides NSH-5 |
[21] | |
|
F. oxysporum NSG-1 |
[21] | |
|
Leptosphaeria |
L. microscopica LF5 |
[13] |
|
Mucor |
M. racemosus NSH-D |
[21] |
|
M. fragilis NSY-1 |
[21] | |
|
Mycoleptodisc |
M. terrestris |
[13] |
|
us |
RF83 | |
|
Paecilomyces |
P. tenuis YS-13 |
[22] |
|
Penicillium |
Penicillium sp. SF142 |
[13] |
|
P. griseofulvum LF146 |
[13] | |
|
Penicillium |
P. polonicum hy4 |
[23] |
|
Penicillium sp. LDL4.4 |
[24] | |
|
Sharaira |
Sharaia sp. Slf14 |
[25] |
|
S. bambusicola LF15 |
[13] | |
|
Trichoderma |
T. harzianum L44 |
[26] |
|
T. harzianum NSW-V |
[21] | |
|
Phlegmariurus phlegmaria as host plant | ||
|
Ceriporia |
C. lacerate MY183 |
[27] |
|
Colletotrichum |
C. gloeosporioides MJ422 C. |
[27] |
|
gloeosporioides MJ484 C. boninense |
[27] | |
|
MY298 C. boninense |
[27] | |
|
MY252 C. guizhouensis |
[27] | |
|
MJ216 |
[27] | |
|
Hypoxylon |
H. investiens MY311 |
[27] |
|
Trichoderma |
T. harzianum MY237 |
[27] |
|
Phlegmariurus taxifolius as host plant | ||
|
Fusarium |
Fusarium sp. C17 |
[28] |
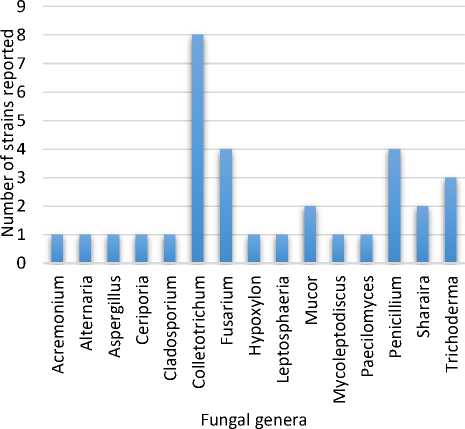
Figure 2. Endophytic fungal strains inhabiting the Huperziaceae family reported capable of producing Hup A
DISCUSSION
Alzheimer's disease (AD) is a progressive, degenerative brain disorder and the most common form of dementia in older adults. AD is associated with a loss of the cholinergic system, with reduced levels of acetylcholine in areas of the brain associated with learning, memory, behavior, and emotional responses.[29] The presence of beta-amyloid (Aβ) plaques, neurofibrillary tangles, and degeneration or atrophy of basal forebrain cholinergic neurons are the main characteristics commonly found in AD. Reduction of synaptic acetylcholine (ACh) availability due to the loss of basal forebrain cholinergic cells could further lead to cognitive impairment in the case of AD.[30]
The neurotransmitter acetylcholine (ACh) is located in sweat glands and the piloerector muscles of the sympathetic autonomic nervous system, specifically at the neuromuscular junction between motor
nerves and skeletal muscles in the peripheral nervous system. ACh functions as a neurotransmitter in all parasympathetic innervated organs. ACh is mainly founded in interneurons in the central nervous system, and several critical long-axon cholinergic pathways have also been identified. Of particular note are the cholinergic projections from the nucleus basalis of Meynert (in the basal forebrain) to the forebrain neocortex and associated limbic structures. The degeneration of this process contributes to AD pathologies.[31] Acetylcholinesterase (AChE) is selectively able to catalyze the ester bond on acetylcholine through hydrolysis at the synaptic cleft to terminate its impulse transmission role.[32] Moreover, in vertebrates, shortly after the release of presynaptic neuronal, AChE modulates cholinergic neurotransmission through the inactivation of acetylcholine.[33]
AChE inhibitors can enhance cholinergic transmission as well as protect brain cells against free radical injury. AChEIs also interfere with aggregation and deposition processes of Aβ protein which are suspected as the second mechanism of AD.[14] AChEIs act by inhibiting the cholinesterase enzyme to breakdown ACh, which eventually increases the level of ACh and prolongs this neurotransmitter action. The action of AChE inhibitors can be irreversible or reversible. Reversible AChE inhibitors, either competitive or noncompetitive inhibition, are mostly therapeutically valuable, whereas toxic effects are frequently related to irreversible AChE inhibitors.[31]
Endophytic fungi are obtained from plant tissues through a series of surface
sterilization processes. The number of endophytic fungi is very abundant and can be found in various plant tissues. Endophytic fungi occupy the inner tissues of the host plants during a particular period or throughout their life stages. They form mutualism relationship with the host plants without causing any detrimental effects to their host.[34] Endophytic fungi can also exist in non-pathogenic and pathogenic phases. Many non-pathogenic endophytes are dormant pathogens, which could be pathogenic under unfavorable conditions or after plant senescence. Therefore, given the nature of their hosts, endophytic fungi could give beneficial effects on particular host plants, whereas they could be found as pathogens to other plants.[14]
As repeatedly reported in the literature, fungal endophytes can provide many fitness benefits to their host as they are biologically active,[35] can enhance the growth of their hosts, and increase host resistance to disease-causing phytopathogens and environmental stress. Interestingly, many fungal endophytes can also produce bioactive metabolites as those found in the host plant. The horizontal gene transfer hypothesis has revealed the ability of endophytes to produce identical bioactive natural products as those afforded from the host plant [14], as exemplified by the production of Hup A from various endophytic fungal strains isolated from the Huperziaceae family. In this case, the horizontal gene transfer is regarded as asexually genetic materials transfer between endophytic fungi and their host plants.[36]
Interestingly, a UV-irradiated strain of an endophytic fungus derived from Lycopodium serratum Thunb. var. longipetiolatum Spring. (synonym of H. serrata) assigned as Paraboeremia sp. Lsl3KI076, was found to be able to biosynthesize Hup A as well, along with
other lycopodium-type of alkaloids.[37] These findings indicate that endophytic fungi possess gene clusters encoded enzymes involved in the biosynthetic pathway of secondary metabolites that were initially reported as plant metabolites, as shown in the production of Hup A by several strains of endophytic fungi. It is well recognized that many gene clusters are “silent” under standard culture conditions, thus various approaches directed to trigger the activation of silent biosynthetic gene clusters can be applied, as exemplified by the production of Hup A by Paraboeremia sp. Lsl3KI076, a UV-irradiated strain of Paraboeremia sp. Lsl3.[37] However, the questions on whether these metabolites are originally biosynthesized by the host plant or were biosynthesized as a result of a mutualistic relationship between the host plant and endophytic fungi inhabiting its internal tissues are yet to be answered.
The biological activity of Hup A related to its potential use in AD therapy has been extensively investigated in many in vitro and in vivo studies, including its mechanism of action as an AChEI. Hup A is a reversible AChEI. Compared to other AChEIs such as galantamine, rivastigmine, and tacrine, Hup A has better penetration through the bloodbrain barrier with higher oral bioavailability and longer AChE inhibition.[31] Hup A can increase the level and duration of action of ACh by preventing its breakdown.[38] The in vitro test showed that Hup A inhibits AChE, with IC50 values of 0.082, 0.093, 0.010, 181.39, and 1.995 µM for Hup A, tacrine, donepezil, rivastigmine, and galanthamine, respectively. This result showed that the AChE inhibitory activity of HupA is equivalent to or even better than the pharmaceutical products currently used in AD therapy.[39] Considering the remarkable activity of Hup A as AChEI, strategy to optimize the capacity of endophytic fungal
stains capable of producing this valuable compound, especially those inhabiting the Huperziaceae plants as shown in Figure 2, will be promising for further investigation.
CONCLUSION
We described herein the capability of endophytic fungal strains associated with Huperziaceae plants to produce Hup A, an active AChEI agent, which could be considered as an alternative producer of Hup A to meet the industrial demand for this pharmaceutically valuable compound. Thirty-two strains of fungal endophytes belonging to fifteen fungal genera were documented as capable of producing Hup A. These fungal endophytes were isolated from Huperziaceae plants included Huperzia serrata, Phlegmariurus phlegmaria and Phlegmariurus taxifolius. Colletotrichum, Fusarium and Penicillium were among the genera of endophytic fungal strains frequently reported as Hup A producers.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
N.P.A. thanks Udayana University for continued support.
REFERENCES
-
1. Ariantari NP, Ancheeva E, Frank M, Stuhldreier F, Meier D, Gröner Y, et al. Didymellanosine, a new
decahydrofluorene analogue, and ascolactone C from: Didymella sp. IEA-3B.1, an endophyte of Terminalia catappa. RSC Adv. 2020; 10 (12):
7232–40.
-
2. Aly AH, Debbab A, Proksch P. Fungal endophytes: Unique plant inhabitants with great promises. Appl Microbiol Biotechnol. 2011; 90 (6): 1829–45.
-
3. Stierle A, Strobel G, Stierle D. of Pacific
Yew. Ecology. 1978; 260 (11): 2–4.
-
4. Puri SG, Verma V, Amna T, Qazi GN, Spiteller M. An endophytic fungus from Nothapodytes foetida that produces camptothecin. J Nat Prod. 2005; 68 (12): 1717–9.
-
5. Bhat BA, Almilaibary A, Mir RA, Aljarallah BM, Mir WR, Ahmad F, et al. Natural Therapeutics in Aid of Treating Alzheimer’s Disease: A Green Gateway Toward Ending Quest for Treating Neurological Disorders. Front Neurosci. 2022; 16 (May): 1–23.
-
6. WHO. Dementia. WHO Press. 2022; https://www.who.int/news-room/fact-sheets/detail/dementia (accessed
November 28, 2022).
-
7. Xiao Y, Liang W, Liu D, Zhang Z, Chang J, Zhu D. Isolation and acetylcholinesterase inhibitory activity of asterric acid derivatives produced by Talaromyces aurantiacus FL15, an endophytic fungus from Huperzia serrata. 3 Biotech. 2022; 12 (3): 1–14.
-
8. Ma X, Tan C, Zhu D, Gang DR, Xiao P. Huperzine A from Huperzia species-An ethnopharmacolgical review. J
Ethnopharmacol. 2007; 113 (1): 15–34.
-
9. Pang B, Yin D, Zhai Y, He A, Qiu L, Liu Q, et al. Diversity of endophytic fungal community in Huperzia serrata from different ecological areas and their correlation with Hup A content. BMC Microbiol. 2022; 22 (1): 1–15.
-
10. Cao D, Sun P, Bhowmick S, Wei Y, Guo B, Wei Y, et al. Secondary metabolites of endophytic fungi isolated from Huperzia serrata. Fitoterapia. 2021; 155 (August): 104970.
-
11. Ma X, Tan C, Zhu D, Gang DR. A survey of potential huperzine A natural resources in China: The Huperziaceae. J Ethnopharmacol. 2006; 104 (1–2): 54– 67.
-
12. Ma XQ, Jiang SH, Zhu DY. Alkaloid
patterns in Huperzia and some related genera of Lycopodiaceae sensu lato occurring in China and their contribution to classification. Biochem Syst Ecol. 1998; 26 (7): 723–8.
-
13. Wang Y, Zeng QG, Zhang Z Bin, Yan RM, Wang LY, Zhu D. Isolation and characterization of endophytic
huperzine A-producing fungi from Huperzia serrata. J Ind Microbiol Biotechnol. 2011; 38 (9): 1267–78.
-
14. Zaki AG, El-Sayed ESR, Abd Elkodous M, El-Sayyad GS. Microbial
acetylcholinesterase inhibitors for Alzheimer’s therapy: recent trends on extraction, detection, irradiation-assisted production improvement and nano-structured drug delivery. Appl Microbiol Biotechnol. 2020; 104 (11): 4717–35.
-
15. Zhang Z Bin, Zeng QG, Yan RM, Wang Y, Zou ZR, Zhu D. Endophytic fungus Cladosporium cladosporioides LF70 from Huperzia serrata produces Huperzine A. World J Microbiol Biotechnol. 2011; 27 (3): 479–86.
-
16. Zhao XM, Wang ZQ, Shu SH, Wang WJ, Xu HJ, Ahn YJ, et al. Ethanol and Methanol Can Improve Huperzine A Production from Endophytic
Colletotrichum gloeosporioides ES026. PLoS One. 2013; 8 (4): 4–12.
-
17. Shu S, Zhao X, Wang W, Zhang G, Cosoveanu A, Ahn Y, et al. Identification of a novel endophytic fungus from Huperzia serrata which produces huperzine A. World J Microbiol Biotechnol. 2014; 30 (12): 3101–9.
-
18. Kang X, Liu C, Shen P, Hu L, Lin R, Ling J, et al. Genomic characterization provides new insights into the biosynthesis of the secondary metabolite huperzine a in the endophyte Colletotrichum gloeosporioides Cg01.
Front Microbiol. 2019; 10 (JAN): 1–15.
-
19. Cui L, Noushahi HA, Zhang Y, Liu J, Cosoveanu A, Liu Y, et al. Endophytic fungal community of huperzia serrata: Diversity and relevance to the production of huperzine a by the plant host. Molecules. 2021; 26 (4): 1–19.
-
20. Le TTM, Hoang ATH, Nguyen NP, Le TTB, Trinh HTT, Vo TTB, et al. A novel huperzine A-producing
endophytic fungus Fusarium sp. Rsp5.2 isolated from Huperzia serrate. Biotechnol Lett. 2020; 42 (6): 987–95.
-
21. Wen-Xia H, Zhong-Wen H, Min J, Han Z, Wei-Ze L, Li-Bin Y, et al. Five novel and highly efficient endophytic fungi isolated from Huperzia serrata expressing huperzine A for the treatment of Alzheimer’s disease. Appl Microbiol Biotechnol. 2020; 104 (21): 9159–77.
-
22. Su J, Yang M. Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata. Nat Prod Res. 2015; 29 (11): 1035–41.
-
23. Kang X, Liu C, Liu D, Zeng L, Shi Q, Qian K, et al. The complete mitochondrial genome of huperzine A-producing endophytic fungus
Penicillium polonicum. Mitochondrial DNA Part B Resour. 2016; 1 (1): 202–3.
-
24. Thi Minh Le T, Thi Hong Hoang A, Thi Bich Le T, Thi Bich Vo T, Van Quyen D, Hoang Chu H. Isolation of
endophytic fungi and screening of Huperzine A–producing fungus from Huperzia serrata in Vietnam. Sci Rep. 2019; 9 (1): 1–13.
-
25. Zhu D, Wang J, Zeng Q, Zhang Z, Yan R. A novel endophytic Huperzine A-producing fungus, Shiraia sp. Slf14, isolated from Huperzia serrata. J Appl Microbiol. 2010; 109 (4): 1469–78.
-
26. Dong LH, Fan SW, Ling QZ, Huang
BB, Wei ZJ. Indentification of huperzine A-producing endophytic fungi isolated from Huperzia serrata. World J Microbiol Biotechnol. 2014; 30 (3): 1011–7.
-
27. Zhang FF, Wang MZ, Zheng YX, Liu HY, Zhang XQ, Wu SS. Isolation and characterzation of endophytic
Huperzine A-producing fungi from Phlegmariurus phlegmaria. Microbiol (Russian Fed. 2015; 84 (5): 701–9.
-
28. Cruz-Miranda OL, Folch-Mallol J, Martínez-Morales F, Gesto-Borroto R, Villarreal ML, Taketa AC. Identification of a Huperzine A-producing endophytic fungus from Phlegmariurus taxifolius. Mol Biol Rep. 2020; 47 (1): 489–95.
-
29. Thompson PA, Wright DE, Counsell CE, Zajicek J. Statistical analysis, trial design and duration in Alzheimer’s disease clinical trials: A review. Int Psychogeriatrics. 2012; 24 (5): 689–97.
-
30. Liu W, Li J, Yang M, Ke X, Dai Y, Lin H, et al. Chemical genetic activation of the cholinergic basal forebrain hippocampal circuit rescues memory loss in Alzheimer’s disease. Alzheimer’s Res Ther. 2022; 14 (1): 1– 20.
-
31. Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM.
Acetylcholinesterase Inhibitors:
Pharmacology and Toxicology. Curr Neuropharmacol. 2013; 11 (3): 315–35.
-
32. Williams P, Sorribas A, Howes MJR. Natural products as a source of Alzheimer’s drug leads. Nat Prod Rep. 2011; 28 (1): 48–77.
-
33. Pope CN, Brimijoin S. Cholinesterases and the fine line between poison and remedy. Biochem Pharmacol. 2018; 153 (January): 205–16.
-
34. Baron NC, Rigobelo EC. Endophytic fungi: a tool for plant growth promotion
and sustainable agriculture. Mycology. 2022; 13 (1): 39–55.
-
35. Ariantari NP, Frank M, Gao Y, Stuhldreier F, Kiffe-Delf AL, Hartmann R, et al. Fusaristatins D–F and (7S,8R)-(-)-chlamydospordiol from Fusarium sp. BZCB-CA, an endophyte of Bothriospermum chinense.
Tetrahedron. 2021; 85.
-
36. Alam B, Lǐ J, Gě Q, Khan MA, Gōng J, Mehmood S, et al. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front Plant Sci. 2021; 12
(December): 1–24.
-
37. Ishiuchi K, Hirose D, Suzuki T, Nakayama W, Jiang WP,
Monthakantirat O, et al. Identification of Lycopodium Alkaloids Produced by an Ultraviolet-Irradiated Strain of
Paraboeremia, an Endophytic Fungus from Lycopodium serratum var.
longipetiolatum. J Nat Prod. 2018; 81 (5): 1143–7.
-
38. Wang J, Chen F, Zheng P, Deng W, Yuan J, Peng B, et al. Huperzine A ameliorates experimental autoimmune encephalomyelitis via the suppression of T cell-mediated neuronal in fl ammation in mice. Exp Neurol. 2012; 236 (1): 79–87.
-
39. Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006; 27 (1): 1–26.
52
Discussion and feedback