DETECTING TRANSOVARIAL INFECTION IN AEDES AEGYPTI BASED ON IMMUNOCYTOCHEMICAL STREPTAVIDIN BIOTIN PEROXIDASE COMPLEX ASSAY (ISBPC) IN BALI
on
Journal of Health Sciences and Medicine, Vol. 2 No. 1, February 2018
DETECTING TRANSOVARIAL INFECTION IN AEDES AEGYPTI BASED ON IMMUNOCYTOCHEMICAL STREPTAVIDIN BIOTIN PEROXIDASE COMPLEX ASSAY (ISBPC) IN BALI
Sang Gede Purnama1, Pasek Kardiwinata1, Tri BaskoroTunggul Satoto2
Email: sangpurnama@unud.ac.id
-
1. School of Public Health, Medicine Faculty, Udayana University
-
2. Parasitology Department, Medicine Faculty, Gajah Mada University
ABSTRACT
Immunocytochemical method is one of dengue virus examination alternative with affordable cost. Through immunocytochemical methods it will be proven that transovarial transmission of dengue virus from mosquitoes to their eggs and its relationship with the incidence of DHF in Bali. This study is done by the installationovitrap indoor and outdoor as much as 1200 points. The mosquito will be cultured for dengue virus examination using the Immunocytochemical-Immunomodoxidase streptavidin biotin complex (IISBC) method of head squash. The results of examination of larvae density were known to the ovitrap index in Denpasar (4.4), Tabanan (6.6), Gianyar (8.9). There is evidence of transovarial transmission from female mosquitoes to eggs using Immunocytochemical Technique known to its transovarial index of each city ie Denpasar (11%), Gianyar (7.14%) and Tabanan (7.14%). Key words: transovarial, immunocytochemical, Bali
-
I. BACKGROUND
Dengue infection is still high in the tropics and some subtropical areas, especially in southeast Asia. An estimated 390 million people are infected with dengue each year and 90 million require medical care [1]. Aedes aegypti and Aedesalbopictus have a major role in the spread that is known to carry dengue virus serotypes (Den 1 to 4) [2].
Monitoring of mosquito populations with egg traps (ovitrap) as well as detection of dengue virus in mosquitoes is important to know the transovarial transmission of dengue virus as well as the number of mosquito densities in this area. This surveillance activity is part of active observation in the context of the implementation of early awareness in endemic areas.
Several studies of transovarial transmission have been done on Aedesalbopictus [3], [4], [5], Aedes aegypti [5], [6].Transmission of dengue virus with transovarial in Indonesia in nature has also been conducted in Klitren, Yogyakarta [7], [8] with immunocytochemical immunopo- toxidase streptavidin biotin complex (IISBC) method of head squash preparation.Study with immunocytochemical
methods in four districts in Central Java [9], found transovarial DHF transmission. The study of several urban villages in Yogyakarta also found vertical transmission in the mosquito body, with infection rate of 38.5% - 70.2% [10].
Methods for virus identification include direct fluorescent antibody (DFA) test on mosquito tissue, usually brain and salivary glands or head squash and reverse transcription polymerase chain reaction (RT-PCR), but the immunoflurosensi method takes longer, require fluorescent and cryofreezer microscopes that are not always available in laboratories whereas RT-PCR requires a more expensive tool.
Another simpler method of immunocytochemical immunoperoxidase streptavidin biotin complex (IISBC) in head squash preparations. This technique, although qualitative but sensitive, is specific and valid for the diagnostic purposes of dengue virus infection in Aedessp mosquitoes [7]. This method is easier and the reagent kit is relatively inexpensive so it can be done in a place with less laboratory facilities.
-
II. METHOD AND PROCEDURES
The type of this study was observational analytic with cross-sectional design. here are 1200 ovitrap placed in indoor and outdoor locations selected in the house that had been infected dengue infection last 1 week from 3 regencies ie 400 in Tabanan regency, 400 in Gianyar regency and 400 in Denpasar city.
Ovitrap surveillance
Ovitrap made of plastic container size 300 ml size of open top 7.8 cm in diameter, the base diameter was 6.5 cm and the container was 9.0 cm in height. The outside in black paint so that the smell of paint is not felt then soaked with water for 1 x 24 hours. then water was added about 5.5 cm [11]. Further placed in indoor and outdoor randomly, ovitrapTheovitraps were collected after 5 days and replaced with fresh ovitrap. Measurement of ovitrap index by knowing the number of positive ovitrap contains eggs. Eggs are then cultured into mosquitoes and then counted the number of population. Furthermore, mosquitoes are cultured into F1 derivatives. F1 then performed dengue virus examination with Immunocytochemical-Immunomodoxidase streptavidin biotin complex (IISBC) method on head squash.
Examination of dengue virus with SBPC immunocytochemical method Tools and materials
Laboratory equipment and materials for the detection of dengue viruses with SBPC immunocytochemical method: Prepared glass, 24X50 mm long covering glass, 200 micro pipette and 10 micro, yellow tip, label paper, methanol absolute, H2O2 (hydrogen peroxide). Ae mosquito. Agypti and Ae. Albopictus colonization results from egg collection from the field. Novostain Universal Detection Kit contains three ready-to-use reagents: Predulated normal horse serum, Predulatedbiotinilated secondary antibody that recognizes rabbit serum (IgG) and serum mice (IgG and IgM), Predulated streptavidin peroxidase conjugate, Other ingredients to be provided: chromogen: diaminobenziden tetrachloride (DAB) .The glass preparations used are glass preparations which have been coated Poly L lysine and should be placed on a moist container during the incubation process eg with a tray covered with wet wipes. Regents should be stored at 2 - 8 0C when not in use.
Preparation of the substance: Peroxidase blocking solution: one part peroxidase 30% plus 9 parts of methanol absolute. PBS BA 0.5% or PBS
containing 5% serum delute blocking (NCL-H serum) to dilute primary antibody. Antibody monoclonal anti-dengue commercial 1: 200. One DAB tablet diluted with 15 ml distilled immediately prior to use.
SBPC immunocytochemical method
The method used is SBPC immunocytochemistry in mosquito histologist preparation on mosquito headprint preparation. Preparation of mosquito headprint preparation with the following procedure [7], [8].
Aedes aegypti 7 days old that has been turned off then made head squash. the head is separated from its body in a specially separated glass. The preparation is fixation with cold methanol 200 C with for 3-5 minutes.Preparations were washed under a tap shortly afterwards with PBS. To remove endogenous peroxidase activity the preparations are immersed in peroxidase blocking solution at room temperature for 10 minutes or under tap water for 5 minutes. Preparations were incubated in prediluted blocking solution for 10 min at room temperature of 250C. The primary antibody of commercially prepared monoclonal antibody is added as much as 100 µl per preparation and then incubated in the refrigerator for 24 hours. The preparation is then washed with PBS for 5 minutes. Biotinylated universal secondary antibody of 100 µl per preparation was added, then the preparation was incubated at room temperature 250 C for 10 min.
The preparation was washed with PBS for 5 minutes. The preparations were incubated with ready-to-use streptavidin / peroxidase complex reagents for 10 minutes. The preparation was washed with PBS for 5 minutes. The preparations were incubated with 100 µl peroxidase substrate solution (DAB) per preparation for 2-10 minutes (the thicker the incubation time was getting longer) The preparations were washed with 100 µl Mayer hematoxylin (counterstain) tap water added, incubated for 1-3 minutes then washed under tap water. The preparation is subsequently dipped into alcohol, cleaned, immersed in xylol. The preparation is then spilled with a mounting medium and then covered with a preparatory glue. After dry the preprat is ready to be examined under a microscope under 400 times magnification and 1000 times. Preparations showing brown color mean positive dengue antigen, whereas preparations that show blue or pale as negative controls do not contain dengue antigens. Each time the coloring should be provided with negative control and positive control. positive controls ie infectious dengue mosquito preparations
that are reacted with primary antibodies. Negative control of noninfectious preparations reacted with primary antibody diluents or noninfectious mosquito preparations treated with primary antibodies.
-
III. RESULT AND DISCUSSION
Ovitrap placed at 600 points inside and 600 dots outside the respondent's house in Denpasar, Gianyar and Tabanan districts. The number of mosquitoes in Gianyar total 3561 iemale 1721 and female 1840, in Denpasar there are 1785 mosquitoes ie males 840 and female 945. In Tabanan there are 2650 mosquitoes ie males 1290 and females 1360. In each region found the average mosquito every house in Denpasar 4.4, in Gianyar 6.6 and Gianyar 8.9. Based on data ovitrap index known Gianyar region highest mosquito density level.
Table 1.
The difference in number of adult aedes aegypti mosquitoes from eggs in ovitrap in 3 regions.
|
Locatio n |
ovitra p |
Aedes aegypti |
Total mosqui to |
Avera ge of each house | |
|
Mal e |
Fema le | ||||
|
Gianya r |
400 |
172 1 |
1840 |
3561 |
8,9 |
|
Denpas ar |
400 |
840 |
945 |
1785 |
4,4 |
|
Tabana n |
400 |
129 0 |
1360 |
2650 |
6,6 |
In this study, the data of ovitrap index shows that female mosquitoes in Gianyar and Tabanan area are higher than Denpasar. The average number of mosquitoes per house is also more in Gianyar and Tabanan compared to Denpasar. Denpasar averages 4.4 mosquitoes in each house while in Gianyar of 8.9 tail and Tabanan 6.6 tail. This condition shows that mosquito density in Gianyar and Tabanan is higher. This has a risk of dengue transmission. The study of ovitrap in Malaysia also found high rates of ovitrap index and Aedes aegypti mosquito breeding behavior in indoor and outdoor Aedesalbopictus [12].
The high level of mosquito density in a region is often due to the large number of containers in the area [13], [14], [15]. So many mosquitoes can breed in the area. Areas with high mosquito density will have a high risk of dengue infection. Chemical control efforts precisely lead to resistance [16]. The use of biological means such as bacillus thuringiensis is recommended [17], [18].
Examination of dengue virus in Aedes aegypti mosquito by using IISBC method.Mosquitoes detected as many as 180 mosquitoes, in each village examined 30 female mosquitoes. Mosquitoes used adult Aedes aegypti mosquitoes of the first derivative (F1) aged on average 7 days of satiety with a 10% sugar solution. Special mosquito positive control and negative control is taken from mosquito of Parasitology Laboratory of Faculty of Medicine UGM. In accordance with the standard IISBC method [7] dengue virus detection is performed starting with material preparation, staining and microscopic examination.
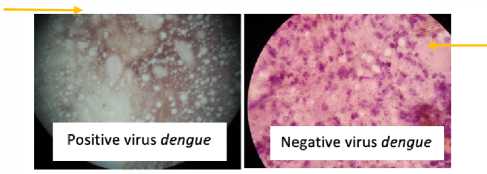
-
Figure 1.
Microscopic images of Aedes aegypti positive Aedes aegypti head squash heads infected virDen and uninfected virDen from the field by the ISBPC method.
In head squash Aedes aegypti mosquito infected with Dengue virus seen positive reaction. Positive reaction is a brown cell cytoplasm and spread among the brain tissue. Negative controls show negative reactions in the form of a blue cell cytoplasm and no brown sand grains around brain tissue cells.
Dengue virus detection results in Aedes aegypti mosquitoes in 3 areas of DHF endemic can be seen in Table below:
Table 2.
Results of transovarial dengue virus transmission using IISBC method in 3 regions
|
No. |
Area |
Total | ||
|
Positive |
Negative |
ITT(%) | ||
|
1 |
Denpasar |
3 |
27 |
11,11 |
|
2 |
Gianyar |
2 |
28 |
7,14 |
|
3 |
Tabanan |
2 |
28 |
7,14 |
The examination results are then calculated transovarial transmission index with the formula below.
jnumber of mosquitoes positive dengue virus
ITT = ----------------------------------X 100%
number of mosquitoes examined
Based on ITT calculation shows that the city of Denpasar has the highest ITT value, ie 11.11% and the incidence of dengue is also highest in Denpasar. Then Gianyar and Tabanan equal to 7.14% and DesaSesetan 7.14%. There is evidence of transovarial transmission of dengue virus in female Aedes aegypti mosquitoes.
Immunocytochemical methods have the ability to identify proteins or antigens in cells and tissues. This method depends on the specificity of the antibody bound to the epitope of the protein used as an immunogen. Specific results relate to 2 criteria ie antibody-specific and methods used [19].
Immunocytochemical examination results found that Denpasar had a dengue virus transovarial index of Aedes aegypti mosquitoes to eggs of 11.1%.This is the highest compared to Gianyar Regency 7.14% and Tabanan 7,14%. The transovarial index shows the presence of vertical dengue transmission from female adult mosquitoes to its eggs. The higher a region has mosquitoes containing the virus the higher the risk of transmission. Denpasar has a relatively low number of mosquito density but the number of mosquitoes containing the virus in this region is greater then the possibility of dengue infection in this region is higher.
Study in Kerala, India also found a natural transovarial transmission [20]. study in Rajasthan, India also found a high rate of transovarial on the Den-3 [21]. the study of persistence of dengue virus shows the amount of transovarial transmission until the seventh generation is relatively stable [22]. Transovarial transmission has been shown to be associated with outbreak occurrences in urban areas [2], [23]. Research in Malaysia shows Aedes aegypti and Aedesalbopictus associated with transovarial transmission with dengue infection in urban areas [24]. Study in Brazil found that Transovarial transmission of DENV was detected in all municipalities. The transovarial infection rate (TOR) in the municipalities was 46% of the DENV positive samples [25].
-
IV. CONCLUSION
The density of mosquitoes based on the ovitrap index in Tabanan and Gianyar areas is higher than that of Denpasar. But the number of mosquitopositive virus in the area of Denpasar more. This causes the case in denpasar to be higher than other regions.The evidence of transovarial transmission from adult mosquitoes to eggs in Denpasar is 11.11% and Gianyar 7.14% and Tabanan 7.14%.
Acknowledgement
The Authors are grateful to appreciate to theMinistry ofResearch, Technology and Higher Education, Republic ofIndonesia, which has funded this research. We are alsograteful to the Institute for Research and CommunityService (LPPM) Udayana University who have contributedproposing this research to be funded.
REFERENCES
-
1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7.
-
2. Thongrungkiat S, Maneekan P, Wasinpiyamongkol L, Prummongkol S.
Prospective field study of transovarial denguevirus transmission by two different forms of Aedes aegypti in an urban area of Bangkok. Thailand J Vector Ecol. 2011;36:147–52.
-
3. Mitchell CJ and Miller BR. Vertical transmission of dengue viruses by strains of Aedesalbopictus recently introduced into Brazil. J Am Mosq Control Assoc. 1990; 6(2): 251253.
-
4. Rosen L, Roseboom LE, Gubler DJ, Lien JC and Chaniotis BN. Comparative susceptibility of mosquito species and strains to oral and parenteral infection with dengue and Japanese encephalitis viruses. Am J Trop Med Hyg. 1985; 34(3): 603-615.
-
5. Rosen L. Further observations on the mechanism of vertical transmission of flaviviruses by Aedes mosquitoes. Am J Trop Med Hyg. 1988; 39(1): 123- 126.
-
6. Chen WJ, Tsai SM, Chen SL, Ko YC and Fang AH. Study on transovarial transmission of dengue type 1 virus in Aedes aegypti. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi XueZaZhi. 1990; 23(4): 259-270.
-
7. Umniyati S.R., Sutaryo, Wahyono D., Artama T, Mardihusodo S J. , Soeyoko, Mulyaningsih B., Utoro T., 2008. Application of monoclonal antibody DSSC7 for detecting dengue infection in Aedes aegypti based on immunocytochemical streptavidin biotin peroxidase complex assay (ISBPC) Dengue Bulletin ; 32: 83-98
-
8. Umniyati, S.R., 2009A. Standardization of immunocytochemical method for the diagnosis of dengue viral infection in Aedes aegypti Linn
mosquitoes (Diptera: Culicidae). BIKed.; 41: p1-10.
-
9. Widiarti, Damar Tri Boewono, UmiWidyastuti.
Deteksi antigen virus dengue pada progeny vector demamberdarah dengue
denganmetodeimunositokimia.
BuletinPenelitiankesehatan. 2009. 37 (3): 126136.
-
10. Mardihusodo, S. J.,. Application non-spesific esterase enzyme microassay to detect potential insecticide resistance of Aedes aegypti adults in Yogyakarta, Indonesia, Berkalailmukedokteran, 1996. 28 (4): 167-171.
-
11. Lee HL. Aedesovitrap and larval survey in several suburban community in Selangor, Malaysia. Mosquito Borne Diseases Bulletin. 1992; 9(1): 9-15.
-
12. Lim Kwee Wee, NorzahiraRaduan, Sing Kong Wah, Wong Hong Ming, Chew Hwai Shi, Firdaus Rambli,a Cheryl
JacylnAhok,aNazniWasi Ahmad, Lee Han Lim, Andrew McKemey& Seshadri Vasan7 Ovitrap surveillance of dengue and chikungunya vectors in several suburban residential areas in Peninsular Malaysia. Dengue bulletin. 2011. 35: 153-161.
-
13. Sugeng J. Mardihusodo, Tri Baskoro T. Satoto, A. Garciab& Dana A. Pupal/demographic and adult aspiration surveys of residential and public sites in Yogyakarta, Indonesia, to inform development of a targeted source control strategy for dengue. Dengue bulletin. 2011. 35: 141-152.
-
14. Sang G Purnama, Tri Baskoro. Maya index dankepadatan larva terhadapinfeksi dengue. J. Makara Kesehatan. 2012. 16 (2): 57-64
-
15. SG Purnama, TB Satoto, Y Prabandari.
Pengetahuan, sikapdanperilakupemberantasansarangnyamukte rhadapinfeksi dengue di Kecamatan Denpasar Selatan. Archive of community health. 2013. 2 (1). 20-27.
-
16. S Purnama, T Baskoro, Y Prabandari. Spatial mapping dengue infection and vulnerability test on Aedes aegypti to organofosfat in South Denpasar District. JurnalKesehatan Masyarakat. 2013. 4 (2): 148-157.
-
17. Hl Santi, SG Purnama. 2014. UjiPatogenitas Bacillus thuringiensis
serovarisraeliensisterhadap larva nyamukAedes sp.
Sebagaibiokontrolpenyebabpenyakitdemamberd arah dengue. Archive of community health. 3 (1): 14-23.
-
18. Sang GedePurnama, Gedesudiana, Denny silvina, 2012. Pemanfaatanlimbahcair industry pengolahantahuuntukmemproduksispora Bacillus thuringiensis. Archive of community health. 1 (1): 1-9.
-
19. Burry RW. Specificity Controls for Immunocytochemical Methods. Journal of Histochem and Cytochem. 2000; 48: 163-166.
-
20. Velayuthamthenmozi, Joghegowder, Satish
Chandra, Paulraj Philip, Rajaiah, Rathinasamy. Natural vertical transmission of dengue virus in Aedesalbopictus (DipteraCulicidae) in Kerala a Southern Indian State. Jpn. J. Infect. Dis. 2007. 60: 245-249.
-
21. Mourya DT, Gokhale, Basu A, Barde PV, Sapkal GN, Padbidri VS, Gore MM. Horizontal and vertical transmission of dengue-2 virus in highly and lowly susceptible strains of Aedes aegypti mosquitoes. 2001. Acta Virol 45: 67– 71.
-
22. Vinod Joshi, D. T. Mourya, and R. C. Sharma persistence of dengue-3 virus through transovarial transmission passage in successive generations of aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg., 2002. 67 (2): 158–161
-
23. Tri BaskoroTunggulSatoto, Siti
RahmahUmniyati, FardhiasihDwiAstuti, NastitiWijayanti, Laurent Gavotte, Christian Devaux, Roger Frutos. Assessment of vertical dengue virus transmission in Aedes aegypti and serotype prevalence in Bantul, Indonesia. Asian Pac J Trop Dis 2014; 4(Suppl 2): S563-S568.
-
24. Lee, H. L. &Rohani A., 2005. Transovarial transmission of dengue virus in Aedes aegypti and Aedesalbopictus in relation to dengue outbreak in an urban area in Malaysia. Dengue bulletin. 29: 106-111.
-
25. Cristiano Fernandes da Costa, Ricardo Augusto dos Passos, José Bento Pereira Lima, Rosemary Aparecida Roque, Vanderson de Souza Sampaio, Thais BonifácioCampolina, NágilaFrancinete Costa Secundino and Paulo FilemonPaolucciPimenta. Transovarial transmission of DENV in Aedes aegypti in the Amazon basin: a local model of xenomonitoring. da Costa et al. Parasites & Vectors (2017) 10:249
17
Discussion and feedback