Finding Antitubercular Leads from Marine-Derived and Entomopathogenic Fung
on

JURNAL FARMASI UDAYANA | pISSN: 2301-7716; eISSN: 2622-4607 | VOL. 12, NO. 1, 2023
https://doi.org/10.24843/JFU.2023.v12.i01.p09
Finding Antitubercular Leads from Marine-Derived and Entomopathogenic Fungi
Ni Luh Putu Indah Suryani1, I Putu Yogi Astara Putra2, I Nonye Treasure Ujam3 dan Ni Putu Ariantari1
-
1 Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Udayana University, Kampus Unud Bukit-Jimbaran, Badung, 80361 Bali, Indonesia
-
2 Master Program in Biology, Faculty of Mathematics and Natural Sciences, Udayana University, Denpasar, Bali, Indonesia
-
3 Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Enugu State University of Science and Technology (ESUT), Enugu State 401105, Nigeria
Reception date of the manuscript: 2023-06-15
Acceptance date of the manuscript: 2023-07-20
Publication date: 2023-08-31
Abstract— The occurrence of Mycobacterium tuberculosis resistance encourages the discovery of new antitubercular candidates. Microbes including fungi and bacteria are well known reservoir for isolating antibiotic compounds, among natural product resources. In this review, we report promising antitubercular compounds from marine-derived and entomopathogenic fungi, reported from 2000 to 2022. A comprehensive literature search was conducted through scientific websites, including Google Scholar, Springer, and Pubmed. This review includes only research publications reporting antitubercular action of well-defined secondary metabolites with MIC values less than 100 M. Eighty-three antitubercular compounds were reported, where 57 of them derived from fungi associated with marine ecosystem. Those fungi belong to 10 fungal genera. The remaining active compounds were isolated from 7 genera of entomopathogenic fungi. Gliotoxin, 12,13-dihydroxy-fumitremorgin, and helvolic acid as well as hirsutellones A-D are among bioactive compounds reported for their remarkable antitubercular activity, which promising to be investigated further in the search of antitubercular leads. Deeper investigation on these compounds might be promising for the the discovery of antitubercular candidates in the future.
Keywords—Antitubercular; entomopathogenic fungi; marine-derived fungi; Mycobaterium tuberculosis; secondary metabolites
Tuberculosis is one of the deadly diseases caused by Mycobacterium, that require long-term antibiotic treatment (Swain et al., 2022). However, the increasing threat of bacterial resistance to current antibiotics has urged researchers to find new and more potent antibiotics, including those against Mycobacterium. The search for new antitubercular compounds from natural products is one of the strategic steps that can be taken. Therefore, research on antitubercular from natural resources is currently being carried out intensively. Based on the report of Bull and Stach (2007), compounds derived from natural products are a potential source for isolating bioactive secondary metabolites promising as lead compounds for various biological activities. Drug candidates from natural products can be isolated from various sources such as plants, animals and microorganisms (Ye et al., 2020). In this review we explore the search for antituber-cular candidates from natural materials produced by microorganisms. Among microbes, fungi are one of well-known
Penulis koresponden: Ni Putu Ariantari, putu_ariantari@unud.ac.id
sources of antibiotics, as exemplified by the finding on penicillin and cephalosporin antibiotics. Fungi can be found in various biotic and abiotic environments (Ameen et al., 2021), indicating their huge distribution in nature which could lead to their high chemical and biological diversities. Exploration of fungi as sources of bioactive natural products has been widely reported. For example, research by Zang et al. (2022) has been reported antimicrobial compounds produced by Aspergillus flavus QQYZ isolated from the mangrove Kandelia candel namely aflaxanthone A. This compound can inhibit Methicillin-Resistant Staphylococcus aureus with MIC value of 12.5 µM. Apart from the marine environment, fungi can also be isolated from animals, such as insects. These fungi are better known as entomopathogenic fungi, a parasitic microorganisms with an ability to infect and kill arthopods (Litwin et al., 2020). A report by Isaka et al. (2012) isolated a potential antimicrobial compound namely torrubiellin B, which capable of inhibiting Candida albicans and Bacillus cereus with IC50 <10 µg mL-1. The compound was isolated from the entomopathogenic fungus, Torrubiella sp. BCC 28517. These studies indicate that marine-derived and ento-mopathogenic fungi could be a potential source for finding lead compounds promising for the development of antituber-
cular agents. Therefore, in this review, we focus on reporting the presence of antitubercular compounds from marine-derived and entomopathogenic fungi.
As for inclusion criteria, we restricted our literature search to original research paper on antitubercular compounds produced by marine-derived and entomophatogenic fungi, published on 2000 to 2022. Moreover, only research papers describing antitubercular activity of well-defined secondary metabolites with MIC values under 100 µM were included in this review. Research papers that do not fulfill the inclusion criteria and reported the antitubercular activity of the crude extract were excluded. The literature search was conducted using online scientific search through Google Scholar, Springer, Pubmed, Science Direct, Elsevier, and MDPI. The following keywords were used for literature search: antitubercu-lar, Mycobacterium tuberculosis, algae-, coral-, mangrove-, deep sea sediment-, sponge-derived fungi, and entomopatho-genic fungi
Based on our literature search, 70 original research articles were obtained. Thirty of those journals fulfill the inclusion criteria. All papers that met the inclusion criteria were used in this literature review. Minimum inhibitory concentration (MIC) values in the original papers will be converted to µM, as the antibiotic-mediated bacterial interactions evoke a biological response in the recipient bacteria in a dose-dependent manner (Bernier and Surette, 2013) and are not dependent on the weight of the compound.
Antitubercular from marine-derived fungi
Marine-derived fungi are well known as promising producer of various bioactive natural products, some of which show remarkable pharmacological activity for future drug development (Zhen Liu et al., 2020). The following sections describe antitubercular compounds from marine sources including algae-, coral-, deep sea sediment-, mangrove-, and sponge derived fungi.
Algae-derived fungi
Algae-derived fungi were reported to produced diverse bioactive metabolites, some of which were confirmed to demonstrate promising antitubercular activities. Three antimy-cobaterial compounds were isolated from algae-derived fungi, as shown in Figure 1. Halogenated bianthrones called neo-bulgarones D (1) and F (2) were isolated from Penicillium ro-seopurpureum (KP1-135C), derived from alga species, Peta-lonia fascia. This alga was collected at the shores of Green’s Point, L’Etete, NB, Canada. Neobulgarones D and F showed moderate antitubercular activity with MIC values of 46.10 and 31.10 µM (Morehouse et al., 2020). Moreover, investigation on azaphilone derivative, sclerotiorin (3), produced by seaweed-associated fungus, Penicillium sp. strain ZJ27, collected in the South China Sea revealed its antitubercular effect through inhibition of protein kinase G (PknG) of Mycobacterium tuberculosis H37Ra with an IC50 value of 76.50 µM (Chen et al., 2017a).
Coral-derived fungi
Coral-derived fungi have proven to be a treasure trove of structurally unique and biologically active secondary metabolites. Eight antimycobaterial compounds were isolated from coral-derived fungi, as shown in Figure 2. Asperversia-mides A (4), B (5), and C (6) were obtained from Aspergillus versicolor CHNSCLM-0063, isolated from Rumphella aggregata in the South China Sea. All isolated compounds showed mild to weak antitubercular activity towards Mycobacterium marinum with MIC values of 23.4, 81.2, and 87.5 µM, respectively (Hou et al., 2019). Furthermore, the fungus Fusarium graminearum SYSU-MS5127 was isolated from an anemone in Laishizhou Island, Shenzhen City, Guangdong Province, China. This fungus produced several bioactive compounds with inhibitory effects on M. tuberculosis protein-tyrosine-phosphatase B (MptpB). Among these active compounds, fusarielin G (7) gave mild activity with an IC50 value of 23.75 µM, while fusarielins M (8) and N (9) possessed potent to weak inhibition with IC50 values of 1.05 and >40.00 µM, respetively (Chen et al., 2021). Another Fusarium spp. PSU-F14 was isolated from Annella sp. in Gorgonian Sea which produced nigrosporin B (10) and anhydro-fusarubin (11). These compounds showed moderate to weak inhibition against M. tuberculosis H37Ra with MIC values of 41.00 and 87.00 µM (Trisuwan et al., 2010).
Deep sea sediment-derived fungi
A large number of fungal species with the ability to produce bioactive compounds were found in deep sea sediments from different regions of the world and also have proven to be treasure secondary metabolite (Yan et al., 2022). The extreme environment creates the great potential to produce natural products with significant biological properties. Nine antimycobaterial compounds were isolated from deep sea sediment-derived fungi, as shown in Figure 3. In particular, Aspergillus sp. SCSIO Ind09F01 was isolated from deep-sea sediment, collected in the South China Sea. This fungus produced gliotoxin (12), 12,13-dihydroxy-fumitremorgin (13), and helvolic acid (14). These compounds revealed remarkable antitubercular activity against M. tuberculosis with IC50 value of 0.03, 0.89, and 2.41 µM, respectively (Luo et al., 2017). Furthemore, polypropionate derivatives, namely fisc-propionates A (15), B (16), C (17), D (18), E (19), and F (20), were obtained from Aspergillus fischeri FS452, isolated from deep-sea sludge of Indian Ocean sediments at 3000 m depth. All compounds exhibited strong to mild inhibition on the MptpB activity with IC50 values ranging from 4.00 until >50.00 µM (Zhaoming Liu et al., 2019).
Mangrove-derived fungi
Mangrove-derived fungi have been proven as a valuable source in the search of structurally and biologically diverse substances (Qiu et al., 2018). Twenty-seven antimycobate-rial compounds were isolated from mangrove-derived fungi, as shown in Figure 4. Mangrove-associated fungi were revealed to produce a vast range of antitubercular metabolites. An endophytic fungus, identified as Diaporthe sp. SYSU-HQ3, was isolated from fresh branch of the mangrove Excoecaria agallocha. This fungus was found to produce isoprenylisoindole type of alkaloids, characterized as diaporisoindole A (21) and tenellone C (22), which showed prominent inhibition against MptpB with IC50 values
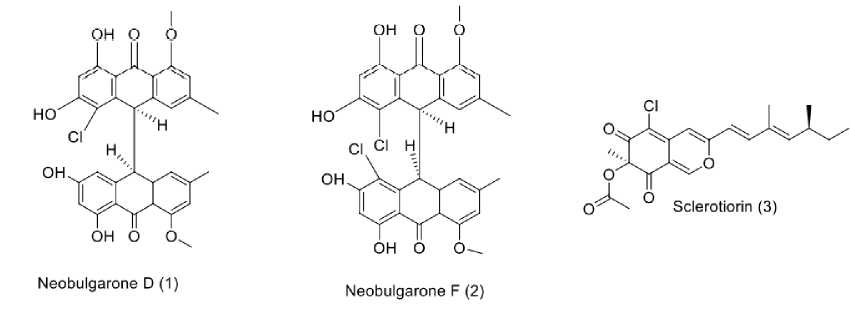
Figure. 1: Structures of compounds (1-3) from algae-derived fungi
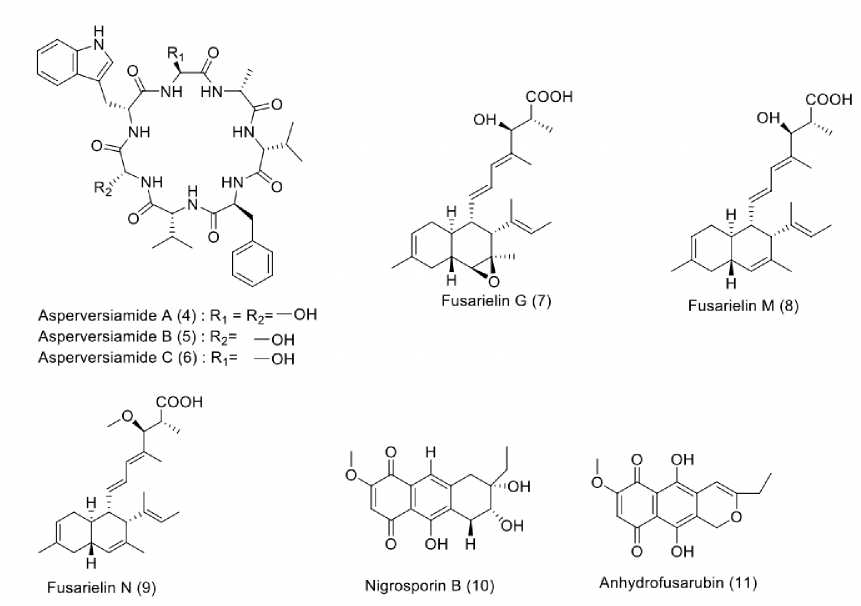
Figure. 2: Structures of compounds (4-11) from coral-derived fungi
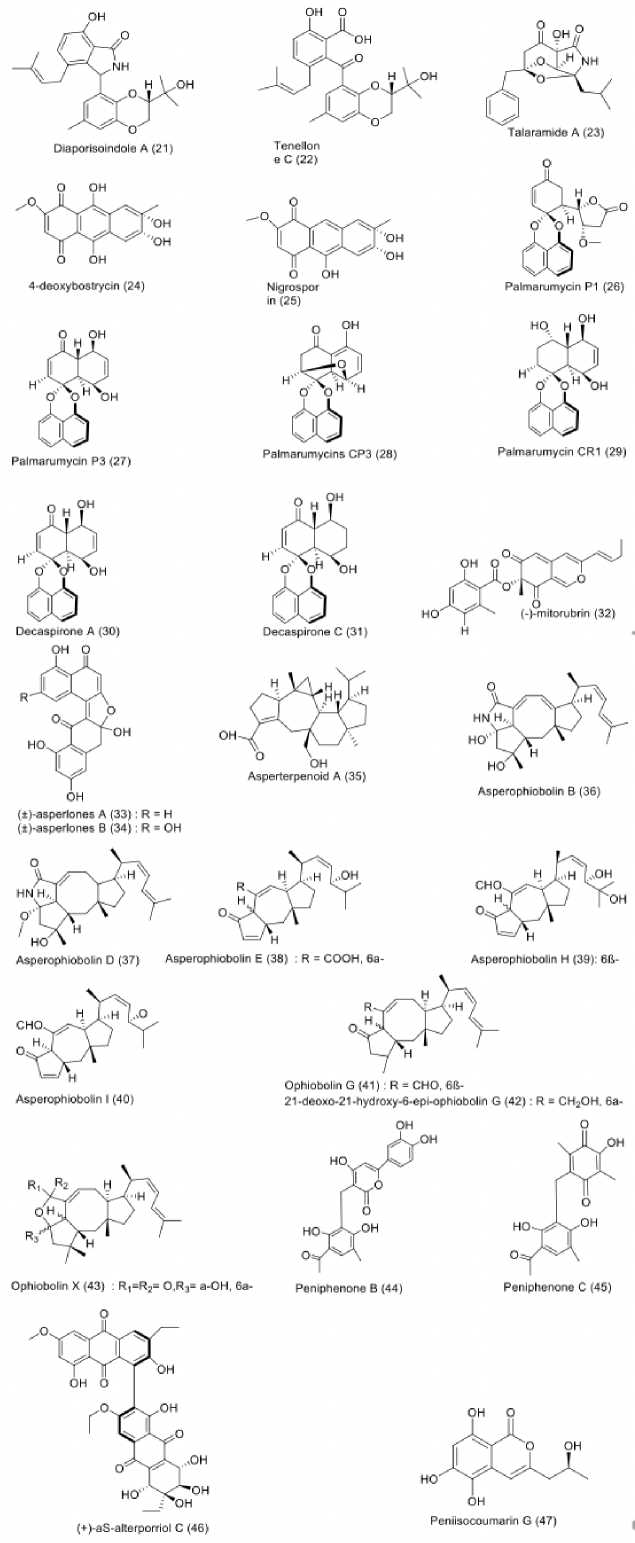
of 4.20 and 5.20 µM (Cui et al., 2017). Moreover, fermentation of another mangrove-associated fungus, Talaromyces sp. (HZ-YX1), isolated from the leaves of Kandelia obo-vata collected in South China Sea, led to the production of alkaloid talaramide A (23), capable of inhibiting mycobacterial PknG activity with an IC50 value of 55.00 µM (Chen et al., 2017b). Furthermore, two anthraquinones, namely 4-deoxybostrycin (24) and nigrosporin (25) were obtained from unidentified mangrove-derived fungus in the South China Sea. These compounds showed inhibition against M. tuberculosis H37Rv with MIC values of 46.83 and 65.72 µM, respectively (Chen et al., 2016). Various naphtalene derivatives were discovered from mangrove-derived fungi as well. Palmarumycins P1 (26), P3 (27), CP3 (28), and CR1 (29), together with decaspirones A (30) and C (31) were obtained from mangrove-associated fungus strain BCC 25093, belonging to a member of the Pleosporales. It was isolated from unidentified mangrove wood in Hat Khanom, Mu Ko Thale Tai National Park, Surat Thani province, Thailand. All isolated compounds showed strong to moderate antitubercu-lar activity with MIC values starting from 1.56 to 12.50 µM (Bunyapaiboonsri et al., 2015). Likewise, three dinaphthale-nones, namely ()-mitorubrin (32) and (±)-asperlones A (33), and B (34) isolated from Aspergillus sp. 16-5C associated with the leaves of Sonneratia apetala collected in Hainan Island, China, strongly inhibited MptpB enzyme with IC50 values of 3.99, 4.24, and 4.32 µM, respectively (Xiao et al., 2015). Moreover, several sesterterpenoid metabolites from Aspergillus species residing in mangrove were also proved to possess antitubercular property. For instance, investigation on Aspergillus sp. 16-5c derived from unidentified mangrove species in South China Sea resulted in the isolation as-perterpenoid A (35), which displayed strong inhibitory activity against MptpB with an IC50 value of 2.20 µM (Huang et al., 2013). In addition, eight ophiobolin-type sesterterpe-noids, namely asperophiobolins B (36), D (37), E (38), H (39), and I (40), ophiobolin G (41), 21-deoxo-21-hydroxy-6-epi-ophiobolin G (42) and ophiobolin X (43) were afforded from Aspergillus sp. ZJ-68 isolated from leaves of Kande-lia candel at Zhanjiang Mangrove Nature Reserve in Guangdong Province, China. Compounds 36-43 showed moderate inhibition against MptpB activity with IC50 values of 19.00 to 42.00 µM, respectively (Cai et al., 2019). A miscellaneous compound from mangrove-derived fungi also exerted antitu-bercular potency. For example, peniphenones B (44) and C (45) from Penicillium dipodomyicola HN4-3A, a fungal endophyte residing in the stem of mangrove species Acanthus ilicifolius, collected in the South China Sea, in Hainan Province, China, showed strong inhibition against MptpB with IC50 values of 0.16 and 1.37 µM, respectively (Li et al., 2014). Additionally, alterporriol-type anthranoid dimer, namely (+)-aS-alterporriol C (46) obtained from Alternaria sp. (SK11) cultures inhibiting root of Excoecaria agallocha, collected in Shankou, Guangxi Province, China, exhibited An-titubercular potency, when evaluated against MptpB, with an IC50 value of 8.70 µM (Xia et al., 2014). Moreover, an isocoumarin, peniisocoumarin G (47), was obtained from Pe-nicillium commune QQF- 3 derived from fruits of Kandelia candel. The mangrove was collected Zhuhai Mangrove Nature Reserve in Guangdong Province, China. Compound 47 moderately inhibited the activity of MptpB enzymes with an
IC50 value of 20.70 µM (Cai et al., 2018).
Sponge-derived fungi
Various natural products have been discovered from sponge-derived fungi in recent years (Sandrawati et al., 2020). Ten antitubercular compounds were isolated from sponge-derived fungi, as shown in Figure 5. A fungus Aspergillus fumigatus MF029, isolated from a marine sponge, Hymeniacidon perleve, collected in Bohai Sea, China, was reported to produce emodin (48), and trypacidin (49). Both isolated compounds showed antitubercular activity against Mycobacterium bovis Bacillus Calmette–Guerin (BCG) with MIC values of 4.63 and 3.63 µM (Song et al., 2021). A number of peptides were also repeatedly isolated from fungi inhabiting sponges. For instance, three trichoderins, namely tri-choderins A (50), A1 (51), and B (52) were obtained from fungus Trichoderma sp. strain 05FI48 isolated from unidentified marine sponge. These trichoderins showed pronounced Antitubercular activity against Mycobacterium smegma-tis (M. sg) with MIC values ranging from 0.09 until 1.36 µM both under aerobic and hypoxic conditions. When tested against M. bovis BCG under aforementioned conditions, these trichoderins gave MIC values below 0.02 µM. Similar result was also shown in an assay against M. tuberculosis H37Rv (Pruksakorn et al., 2010), suggesting the promising antitubercular pharmacophore of trichoderin derivatives. Other peptides, belonging to the aspochracin-type cyclic tripeptides, namely sclerotiotides M (53) and N (54), isolated from Aspergillus insulicola HDN151418 associated with an unidentified sponge found at 410 m depth of Prydz Bay, Antarctica. These peptides showed antitubercular activity against M. phlei with MIC values of 3.13 and 12.50 µM, respectively (Sun et al., 2020). Additionally, several an-titubercular polyketides were also discovered from sponge-derived fungi. A polyketide butyrolactone I (55), was afforded upon chemical investigation on Aspergillus terreus SC-SIO 41008, isolated from sponge Callyspongia sp. collected from the seaside in Xuwen County, Guangdong Province, China. Butyrolactone I showed strong inhibitory activity against MptpB with an IC50 value of 12.03 µM (Thakur et al., 2015). Moreover, two aromatic polyketides, elucidated as isochaetochromin B2 (56), and ustilaginoidin D (57), were isolated from sponge-associated fungus, Metarhizium aniso-pliae mxh-99, collected in Naozhou island, Guangxi Province, China. These compounds showed weak inhibition to the growth of M. phlei with MIC value of 91.49 µM for both of them (Kong et al., 2013).
Antitubercular from entomopathogenic fungi
Insect pathogenic fungi refer to fungi which develop pathogenic relationship with their host insects, the so called entomopathogenic fungi. These fungi can directly infect and kill insects by transgressing their cuticle (Hu Bidochka, 2021). Twenty-six compounds with antitubercular activity were recovered from insect-derived fungi, as shown in Figure 6. Recent study reported insects-associated fungus, Ascher-sonia confluens BCC53152, isolated from Hala-Bala Wildlife Sanctuary in Narathiwat province, Thailand. An alkaloid, terpendole C (58), was afforded from this fungus and found to be moderately active against M. tuberculosis with MIC value of 48.10 µM (Sadorn et al., 2020). More alkaloids were recovered upon the fermentation of Verticillium hemipterige-
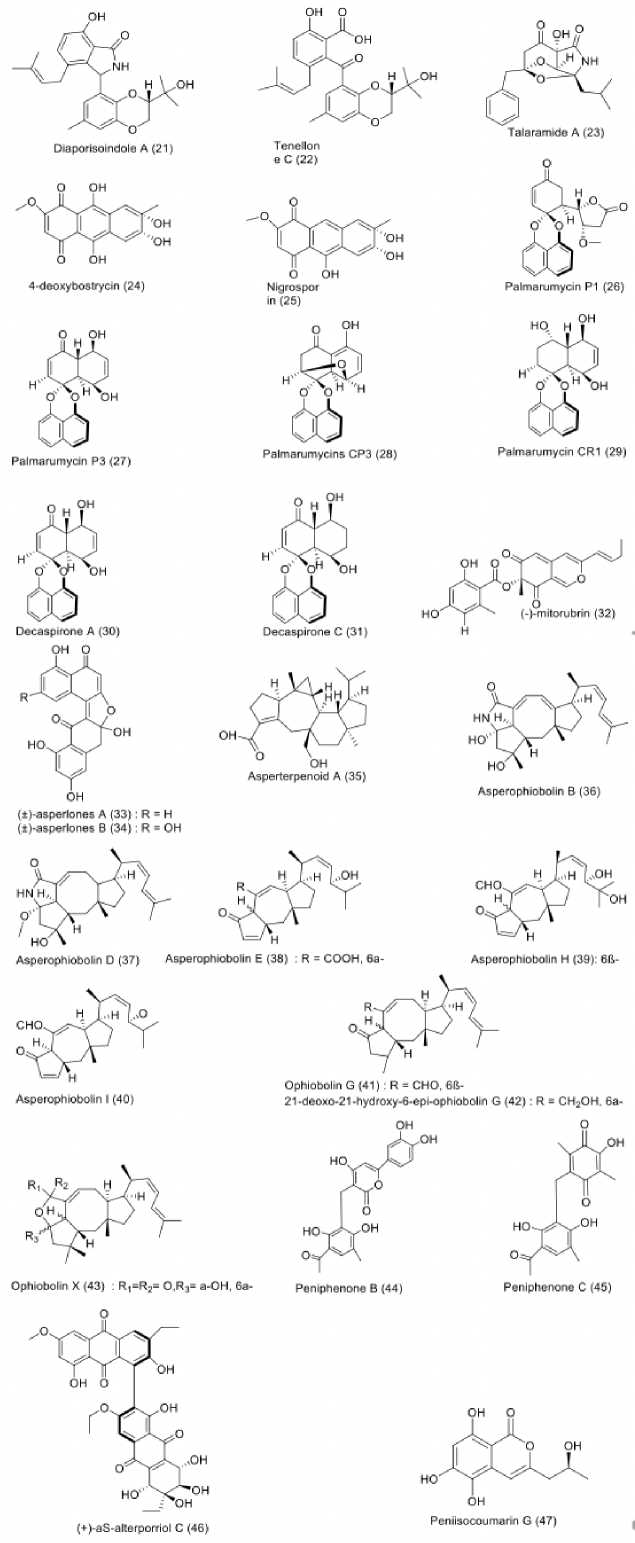
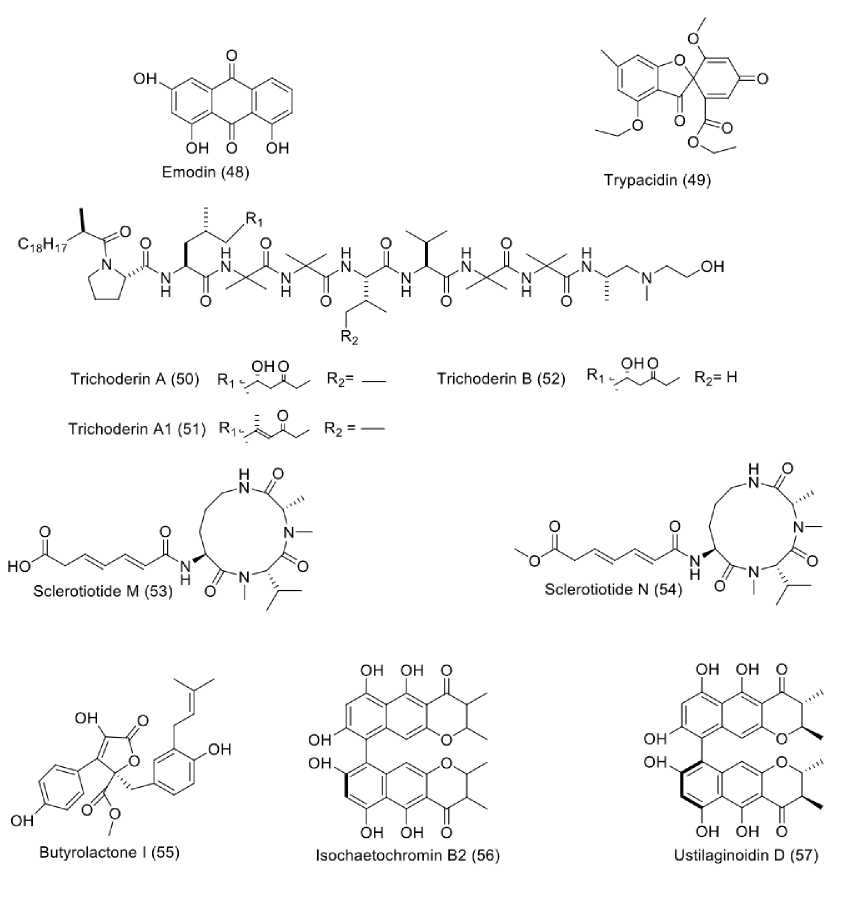
Figure. 5: Structures of compounds (48-57) from sponge-derived fungi.
num BCC 1449, an insect pathogenic fungus isolated from an adult leaf hopper of the Homoptera suborder, collected in Thailand. This fungus naturally synthesized diketopiperazine dimers, vertihemiptellides A (59) and B (60) which had moderate antitubercular activity with MIC values of 20.14 and 20.59 µM (Isaka et al., 2005a). Insect pathogenic fungi were also found to produce a number of antitubercular peptides, as reported by Nilanonta et al. (2000, 2002). This research group investigated a fungus Paecilomyces tenuipes BCC 1614 collected from Khlong Naka Wildlife Sanctuary, Ranong province, southern Thailand. From this fungus, they successfully isolated beauvericin (61), which was reported to have antitubercular activity against M. tuberculosis H37Ra with MIC value of 15.95 µM (Nilanonta et al., 2000). Further research on this fungus revealead that more beauvericins were afforded, including beauvericins A (62) and B (63) along with allobeauvericins A (64), B (65), and C (66). All compounds exhibited potent antitubercular activity with MIC values 2.00 µM (Nilanonta et al., 2002). Previously mentioned V. hemipterigenum BCC 1449 was also capable of producing enniatin derivatives, of which MK1688 (67) was the most
active among the seven isolated enniatins in an assay against M. tuberculosis, with MIC value of 2.21 µM. Meanwhile, enniatins B (68), B4 (69), C (70) G (71), H (72), and I (73) were found to have lower activity with MIC values of 3.259.00 µM (Nilanonta et al., 2003). Moreover, other peptide was also found from Ophiocordyceps communis BCC 16475, an insect-associated fungus isolated from Isoptera collected in Khao Yai National Park, Nakhon Nayok Province, Thailand. Investigation of this fungus resulting in the isolation of cordycommunin (74), which capable of inhibiting the growth of M. tuberculosis H37Ra with MIC value of 15.00 µM (Ha-ritakun et al., 2010). Another insect pathogenic fungi, namely Hirsutella nivea Hywel-Jones BCC 2594 was isolated from homoptera-leafhopper in Khao Yai National Park, Central Thailand. This fungus was reported to produce hirsute-llones A (75), B (76), C (77), and D (78). Following an-titubercular assay against M. tuberculosis H37Ra, all compounds were found to have strong activity with MIC values of 1.75, 1.74, 1.69, and 6.79 µ M (Isaka et al., 2005b). Triterpenoids were isolated from insect pathogenic fungus Ascher-sonia tubulata BCC 1785. This fungus was collected from
Trang Province, Thailand and produced 3β -acetoxy-15α,22-dihydroxyhopane (79), and dustanin (80) with antitubercular activities against M. tuberculosis H37Ra with MIC values of 24.86 and 28.11 µM (Boonphong et al., 2001). Moreover, YM187781 (81), bislunatin (82), and morakotin C (83) were obtained from Cordyceps morakotii BCC 56811. The fungus was isolated from ant (Hymenoptera), collected in Evergreen Forest in Chiang Mai Province, Thailand. These compounds were found to have moderate activity against M. tuberculosis H37Ra with MIC values of 42.66, 43.82 and 82.99 µM (Wang et al., 2019).
This review highlights secondary metabolites from marine-derived and entomopathogenic fungi as promising source for the discovery and development of antitubercular candidates. Among the eighty-three metabolites described herein, several compounds showed remarkable antitubercu-lar activity with MIC values less than 10 µM, including en-niatin derivatives, fusarielin M, gliotoxin, 12,13-dihydroxy-fumitremorgin, hirsutellones A–D, palmarumycins P1 and CP3, as well as trichoderins A and B. Investigation of these metabolites to understand their mode of actions as well as development of their structurally related compounds will be promising for future studies. Moreover, studies on the biosynthetic gene clusters involved in the production of these metabolites by fungal species belonging to Aspergillus, Fusarium, Hirsutella and Trichoderma will enhance our understanding to the capability of these fungi as producer of antitubercular compounds, which may lead to the discovery of more effective antitubercular candidates.
Bernier, S. P., Surette, M. G. (2013). Concentrationdependent activity of antibiotics in natural environments. Frontiers in Microbiology, 4(FEB), 2–3. https://doi.org/10.3389/fmicb.2013.00020
Boonphong, S., Kittakoop, P., Isaka, M., Palittapongarnipim, P., Jaturapat, A., Danwisetkanjana, K., Tanticharoen, M., Thebtaranonth, Y. (2001). A New Antimycobac-terial, 3b- Acetoxy-1 5a,22-dihydroxyhopane, from the Insect Pathogenic Fungus Aschersonia tubulata. Planta Med, 67, 279–281.
Bull, A. T., Stach, J. E. M. (2007). Marine actinobacteria: new opportunities for natural product search and discovery. Trends in Microbiology, 15(11), 491–499. https://doi.org/10.1016/j.tim.2007.10.004
Bunyapaiboonsri, T., Yoiprommarat, S., Nopgason, R., Intereya, K., Suvannakad, R., Sakayaroj, J. (2015). Palmarumycins from the mangrove fungus BCC 25093. Tetrahedron, 71(34), 5572–5578. https://doi.org/10.1016/j.tet.2015.06.061
Cai, R., Jiang, H., Mo, Y., Guo, H., Li, C., Long, Y., Zang, Z., She, Z. (2019). Ophiobolin-Type Sesterterpenoids from the Mangrove Endophytic Fungus Aspergillus sp. ZJ-68. Journal of Natural Products, 82(8), 2268–2278. https://doi.org/10.1021/acs.jnatprod.9b00462
Cai, R., Wu, Y., Chen, S., Cui, H., Liu, Z., Li, C., She, Z. (2018). Peniisocoumarins A-J: Isocoumarins from Penicillium commune QQF-3, an Endophytic Fungus of the Mangrove Plant Kandelia can-del. Journal of Natural Products, 81(6), 1376–1383.
https://doi.org/10.1021/acs.jnatprod.7b01018
Chen, Dong-ni, Chen, H., She, Z., Lu, Y. (2016). Identification of Bostrycin Derivatives as Potential Inhibitors of Mycobacterium tuberculosis Protein Tyrosine Phosphatase (MptpB). Medicinal Chemistry, 12(3), 296–302. https://doi.org/10.2174/1573406411666151005105857
Chen, Dongni, Liu, L., Lu, Y., Chen, S. (2021). Identification of fusarielin M as a novel inhibitor of Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB). Bioorganic Chemistry, 106(November), 104495. https://doi.org/10.1016/j.bioorg.2020.104495
Chen, Dongni, Ma, S., He, L., Yuan, P., She, Z., Lu, Y. (2017a). Sclerotiorin inhibits protein kinase G from Mycobacterium tuberculosis and impairs mycobacterial growth in macrophages. Tuberculosis, 103, 37–43. https://doi.org/10.1016/j.tube.2017.01.001
Chen, S., He, L., Chen, D., Cai, R., Long, Y., Lu, Y., She, Z. (2017b). Talaramide A, an unusual alkaloid from the mangrove endophytic fungus: Talaromy-ces sp. (Hz-YX1) as an inhibitor of mycobacterial PknG. New Journal of Chemistry, 41(11), 4273–4276. https://doi.org/10.1039/c7nj00059f
Cui, H., Lin, Y., Luo, M., Lu, Y., Huang, X., She, Z. (2017). Diaporisoindoles A-C: Three Isoprenylisoindole Alkaloid Derivatives from the Mangrove Endophytic Fungus Diaporthe sp. SYSU-HQ3. Organic Letters, 19(20), 5621–5624. https://doi.org/10.1021/acs.orglett.7b02748
Haritakun, R., Sappan, M., Suvannakad, R., Tasanathai, K., Isaka, M. (2010). An antimycobacterial cyclodepsipep-tide from the entomopathogenic fungus Ophiocordy-ceps communis BCC 16475. Journal of Natural Products, 73(1), 75–78. https://doi.org/10.1021/np900520b
Hou, X. M., Liang, T. M., Guo, Z. Y., Wang, C. Y., Shao, C. L. (2019). Discovery, absolute assignments, and total synthesis of asperversiamides A-C and their potent activity against: Mycobacterium mari-num. Chemical Communications, 55(8), 1104–1107. https://doi.org/10.1039/c8cc09347d
Hu, S., Bidochka, M. J. (2021). Root colonization by endophytic insect-pathogenic fungi. Journal of Applied Microbiology, 130(2), 570–581. https://doi.org/10.1111/jam.14503
Huang, X., Huang, H., Li, H., Sun, X., Huang, H., Lu, Y., Lin, Y., Long, Y., She, Z. (2013). Asperterpenoid A, a new sesterterpenoid as an inhibitor of Mycobacterium tuberculosi s protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Organic Letters, 15(4), 721–723. https://doi.org/10.1021/ol303549c
Isaka, M., Palasarn, S., Rachtawee, P., Vimuttipong, S., Kongsaeree, P. (2005a). Unique diketopiperazine dimers from the insect pathogenic fungus Verticillium hemipterigenum BCC 1449. Organic Letters, 7(11), 2257–2260. https://doi.org/10.1021/ol0507266
Isaka, M., Palasarn, S., Tobwor, P., Boonruangprapa, T., Ta-sanathai, K. (2012). Bioactive anthraquinone dimers from the leafhopper pathogenic fungus Torrubiella sp. BCC 28517. Journal of Antibiotics, 65(11), 571–574. https://doi.org/10.1038/ja.2012.76
Isaka, M., Rugseree, N., Maithip, P., Kongsaeree, P., Prab-pai, S., Thebtaranonth, Y. (2005b). Hirsutellones A-E,
antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea BCC 2594. Tetrahedron, 61(23), 5577–5583. https://doi.org/10.1016/j.tet.2005.03.099
Kong, X., Ma, X., Xie, Y., Cai, S., Zhu, T., Gu, Q., Li, D. (2013). Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their anti-tubercular activities. Archives of Pharmacal Research, 36(6), 739–744. https://doi.org/10.1007/s12272-013-0077-7
Li, H., Jiang, J., Liu, Z., Lin, S., Xia, G., Xia, X., Ding, B., He, L., Lu, Y., She, Z. (2014). Peniphenones AD from the mangrove fungus Penicillium dipodomyico-la HN4-3A as inhibitors of mycobacterium tuberculosis phosphatase MptpB. Journal of Natural Products, 77(4), 800–806. https://doi.org/10.1021/np400880w
Litwin, A., Nowak, M., Róz˙alska, S. (2020). Entomo-pathogenic fungi: unconventional applications. Reviews in Environmental Science and Biotechnology, 19(1), 23–42. https://doi.org/10.1007/s11157-020-09525-1
Liu, Zhaoming, Wang, Q., Li, S., Cui, H., Sun, Z., Chen, D., Lu, Y., Liu, H., Zhang, W. (2019). Polypropionate Derivatives with Mycobacterium tuberculosis Protein Tyrosine Phosphatase B Inhibitory Activities from the Deep-Sea-Derived Fungus Aspergillus fischeri FS452. Journal of Natural Products, 82(12), 3440–3449. https://doi.org/10.1021/acs.jnatprod.9b00834
Liu, Zhen, Frank, M., Yu, X., Yu, H., Tran-Cong, N. M., Gao, Y., Proksch, P. (2020). Secondary Metabolites from Marine-Derived Fungi from China. In Progress in the chemistry of organic natural products, 111. https://doi.org/10.1007/978-3-030-37865-32
Luo, X., Zhou, X., Lin, X., Qin, X., Zhang, T., Wang, J., Tu, Z., Yang, B., Liao, S., Tian, Y., Pang, X., Kaliyaperumal, K., Li, J. L., Tao, H., Liu, Y. (2017). Antituberculosis compounds from a deepsea-derived fungus Aspergillus sp. SCSIO Ind09F01. Natural Product Research, 31(16), 1958–1962.
https://doi.org/10.1080/14786419.2016.1266353
Morehouse, N. J., Flewelling, A. J., Johnson, J. A., Gray, C. A. (2020). Halogenated Bianthrones From Penicillium roseopurpureum: a Fungal Endophyte of the Marine Alga Petalonia fascia. Natural Product Communications, 15(1). https://doi.org/10.1177/1934578X20901405
Nilanonta, C., Isaka, M., Kittakoop, P., Palittapongarn-pim, P., Kamchonwongpaisan, S., Pittayakhajonwut, D., Tanticharoen, M., Thebtaranonth, Y. (2000). An-timycobacterial and antiplasmodial cyclodepsipeptides from the insect pathogenic fungus Paecilomyces te-nuipes BCC 1614. Planta Medica, 66(8), 756–758. https://doi.org/10.1055/s-2000-9776
Nilanonta, Chongdee, Isaka, M., Chanphen, R., Thong-orn, N., Tanticharoen, M., Thebtaranonth, Y. (2003). Unusual enniatins produced by the insect pathogenic fungus Verticillium hemipterigenum: Isolation and studies on precursor-directed biosynthesis. Tetrahedron, 59(7), 1015–1020. https://doi.org/10.1016/S0040-4020(02)01631-9
Nilanonta, Chongdee, Isaka, M., Kittakoop, P., Trakul-naleamsai, S., Tanticharoen, M., Thebtaranonth, Y. (2002). Precursor-directed biosynthesis of beauvericin analogs by the insect pathogenic fungus Paecilomyces
tenuipes BCC 1614. Tetrahedron, 58(17), 3355–3360. https://doi.org/10.1016/S0040-4020(02)00294-6
Pruksakorn, P., Arai, M., Kotoku, N., Vilchze, C., Baughn, A. D., Moodley, P., Jacobs, W. R., Kobayashi, M. (2010). Trichoderins, novel aminolipopeptides from a marine sponge-derived Trichoderma sp., are active against dormant mycobacteria. Bioorganic and Medicinal Chemistry Letters, 20(12), 3658–3663. https://doi.org/10.1016/j.bmcl.2010.04.100
Qiu, L., Wang, P., Liao, G., Zeng, Y., Cai, C., Kong, F., Guo, Z., Proksch, P., Dai, H., Mei, W. (2018). New eudesmane-type sesquiterpenoids from the mangrove-derived endophytic fungus penicillium sp. J-54. Marine Drugs, 16(4), 1–9. https://doi.org/10.3390/md16040108
Sadorn, K., Saepua, S., Punyain, W., Saortep, W., Choo-wong, W., Rachtawee, P., Pittayakhajonwut, P. (2020). Chromanones and aryl glucoside analogs from the entomopathogenic fungus Aschersonia con-fluens BCC53152. Fitoterapia, 144(May), 104606. https://doi.org/10.1016/j.fitote.2020.104606
Sandrawati, N., Hati, S. P., Yunita, F., Putra, A. E., Ismed, F., Tallei, T. E., Hertiani, T., Handayani, D. (2020). Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. Journal of Applied Pharmaceutical Science, 10(4), 28–33. https://doi.org/10.7324/JAPS.2020.104005
Song, Z., Liu, Y., Gao, J., Hu, J., He, H., Dai, S., Wang, L., Dai, H., Zhang, L., Song, F. (2021). Anti-tubercular metabolites from the marine-derived fungus strain Aspergillus fumigatus MF029. Natural Product Research, 35(16), 2647–2654. https://doi.org/10.1080/14786419.2019.1660331
Sun, C., Zhang, Z., Ren, Z., Yu, L., Zhou, H., Han, Y., Shah, M., Che, Q., Zhang, G., Li, D., Zhu, T. (2020). Antibacterial Cyclic Tripeptides from Antarctica-Sponge-Derived Fungus Aspergillus insulicola HDN151418. Marine Drugs, 18(11). https://doi.org/10.3390/MD18110532
Swain, S. S., Pati, S., Hussain, T. (2022). Quinoline heterocyclic containing plant and marine candidates against drug-resistant Mycobacterium tuberculosis: A systematic drug-ability investigation. European Journal of Medicinal Chemistry, 232(March), 1–7. https://doi.org/10.1016/j.ejmech.2022.114173
Thakur, J. P., Haider, R., Singh, D. K., Kumar, B. S., Va-sudev, P. G., Luqman, S., Kalra, A., Saikia, D., Negi, A. S. (2015). Bioactive isochromenone isolated from Aspergillus fumigatus, endophytic fungus from Ba-copa monnieri. Microbiology Research, 6(1), 14–18. https://doi.org/10.4081/mr.2015.5800
Trisuwan, K., Khamthong, N., Rukachaisirikul, V., Phong-paichit, S., Preedanon, S., Sakayaroj, J. (2010). Anthraquinone, cyclopentanone, and naphthoquinone derivatives from the sea fan-derived fungi Fusarium spp. PSU-F14 and PSU-F135. Journal of Natural Products, 73(9), 1507–1511. https://doi.org/10.1021/np100282k
Wang, M., Kornsakulkarn, J., Srichomthong, K., Feng, T., Liu, J. K., Isaka, M., Thongpanchang, C. (2019). Antimicrobial anthraquinones from cultures of the ant pathogenic fungus Cordyceps morakotii BCC 56811. Journal of Antibiotics, 72(3), 141–147.
https://doi.org/10.1038/s41429-018-0135-y
Xia, G., Li, J., Li, H., Long, Y., Lin, S., Lu, Y., He, L., Lin, Y., Liu, L., She, Z. (2014). Alterporriol-type dimers from the mangrove endophytic fungus, Alternaria sp. (SK11), and their MptpB inhibitions. Marine Drugs, 12(5), 2953–2969. https://doi.org/10.3390/md12052953
Xiao, Z., Lin, S. O. E., Tan, C., Lu, Y., He, L., Huang, X., She, Z. (2015). Asperlones a and B, dinaphthale-none derivatives from a mangrove endophytic fungus Aspergillus sp. 16-5C. Marine Drugs, 13(1), 366–378. https://doi.org/10.3390/md13010366
Yan, L. H., Li, X. M., Chi, L. P., Li, X., Wang, B. G. (2022). Six new antimicrobial metabolites from the deep-sea sediment-derived fungus Aspergillus fumigatus SD-406. Marine Drugs, 20(1), 1–14. https://doi.org/10.3390/md20010004
Ye, L., Zhang, J., Xiao, W., Liu, S. (2020). Efficacy and mechanism of actions of natural antimicrobial drugs. Pharmacology and Therapeutics, 216, 32916205. https://doi.org/10.1016/j.pharmthera.2020.107671
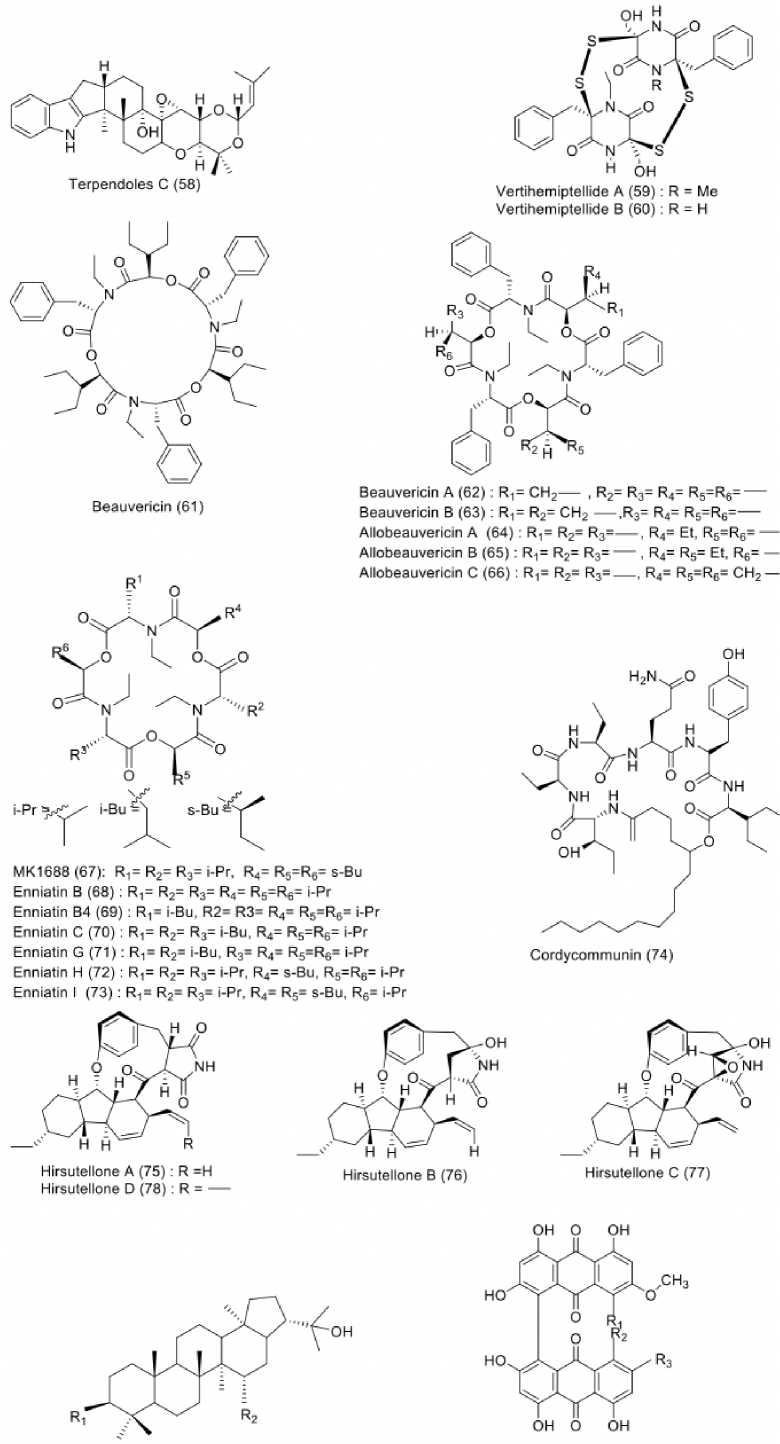
3β- acetoxy-1 5a,22-dihydroxyhopane (79): R1= OAcr R2= OH YM187781 (81): R1= OH, R2-H. R3 - OCH1
Dustanin(BO): R1=H1R2 = OH Bislunatin (B2): R1 = R2=H1R3=OCH1
Morakotin C (83): R1= R2=OH1 R3= O—
Figure. 6: Structures of compounds (58-83) from insect-derived fungi
ARIANTARI ET AL.
62
Discussion and feedback