DAMMARANE-TYPE TRITERPENOIDS FROM THE STEMBARK OF CHISOCHETON PENTANDRUS (MELIACEAE)
on
JURNAL KIMIA (JOURNAL OF CHEMISTRY) 14 (1), JANUARI 2020 DOI: https://doi.org/10.24843/JCHEM.2020.v14.i01.p15
p-ISSN 1907-9850
e-ISSN 2599-2740
DAMMARANE-TYPE TRITERPENOIDS FROM THE STEMBARK OF CHISOCHETON PENTANDRUS (MELIACEAE)
D. G. Katja1*, S. Salam2, Nurlelasari2, D. Harneti2, R. Maharani2,3, U. Supratman2,3 and Y. Shiono4
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Sam Ratulangi University, Kampus Kleak, Manado, 95115, North Sulawesi, Indonesia 2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Indonesia.
3Central Laboratory, Universitas Padjadjaran, Jatinangor 45363, Indonesia. 4Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan
* E-mail: dewakatja@yahoo.com
ABSTRAK
Dua senyawa triterpenoid tipe damaran, cabraleadiol (1) dan cabraleahidrosilakton (2), telah diisolasi dari ekstrak n-heksana kulit batang Chisocheton pentandrus (Meliaceae). Struktur kimia senyawa 1 dan 2 diidentifikasi berdasarkan data-data spektroskopi terutama, NMR dan massa serta perbandingan data spektra dari laporan sebelumnya. Senyawa 1 dan 2 pertama kali dilaporkan pada tumbuhan Chisocheton pentandrus.
Kata kunci: Cabraleadiol, Cabraleahidrosilakton, Chisocheton pentandrus, Meliaceae.
ABSTRACT
Two dammarane-type triterpenoids, cabraleadiol (1) and cabraleahydroxylactone (2), have been isolated from n-hexane extract of the stembark of Chisocheton pentandrus (Meliaceae). The structure of compounds 1 and 2 were determined by spectroscopic data mainly NMR and mass as well as by comparing with previously reported spectral data. Compounds 1 and 2 were reported for the first time from Chisocheton pentandrus.
Keywords: Cabraleadiol, Cabraleahydroxylactone, Chisocheton pentandrus, Meliaceae.
INTRODUCTION
Triterpenoids are the most important group of terpenoids because they exhibit a great diversity of biological activities and they are the major constituents of tropical higher plants. Recently, research of new biologically active compounds from plants used in traditional medicine has led to the isolation of numerous triterpenoids with important biological activities (Fu et al., 2015; Nguyen et al., 2015). Higher plants are major source of triterpenoids with several biological activities and numerous reports have shown that family of Meliaceae, Rhamnaceae, Cucurbitaceae, Ganodermataceae and Apocynaceae produce a wide variety of tetracyclic triterpenoids, whereas, family of Ranunculaceae, Burseraceae, Capparidaceae, Celastraceae and Lamiaceae families are recognized to contain
active pentacyclic triterpenoids (Harneti et al., 2012; Farabi et al 2017; Tian et al., 2005; Zhang et al., 2005).
Chisocheton genera is belong to the Meliaceae family, which consist of more than 50 plant species distributed mainly in tropical countries (Yang et al., 2009; Heyne, 1982). Previous phytochemical studies on Chisocheton species have yielded a number of interesting compounds, including limonoids (Supratman et al., 2019; Supriatno et al., 2018; Katja et al., 2016) and triterpenoids (Inada et al., 1993; Katja et al., 2017 ).
In our continous search for novel constituents from Chisocheton pentandrus, we isolated new limonoids, pentandricin A from the stembark of C. pentandrus (Suprianto et al., 2018). In the further investigation for novel compounds from non polar fraction of C. pentandrus, we found two dammarane-type
triterpenoids from the n-hexane extract. In this paper, we report the isolation and structural determination of two dammarane-type triterpenoids, cabraleadiol (1) and cabraleahydroxylactone (2).
MATERIAL AND METHODS
Plant material
The stem bark of C. pentandrus were collected in Bogor Botanical Garden, Bogor, West Java Province, Indonesia in June 2016. The plant was identified by Mr. Ismail, the staff of the Herbarium and a voucher specimen (No. Bo-104) was deposited at the Herbarium.
Extraction and isolation
The dried ground stembark (1.8 kg) of C. pentandrus was extracted with methanol (3 x 4 L) at room temperature for 6 days. After removal of the solvent under vacuum, the viscous concentrate of methanol extract (340 g) was first suspended in water and then partitioned with n-hexane, ethyl acetate and n-butanol, successively. Evaporation resulted in the crude extracts of n-hexane (10.9 g), ethyl acetate (25.2 g) and n-butanol (228.6 g), respectively. The n-hexane extract (10.9 g) was fractionated by column chromatography on silica gel using a gradient n-hexane-ethyl acetate-methanol to give eight fractions (I– VIII). Fraction II (5.4 g) was subjected to column chromatography over silica gel using a gradient mixture of n-hexane-dichloromethane-ethyl acetate (5% stepwise) as eluting solvents to afford thirteen subfractions (II1-II13). Subfraction II9 (912.2 mg) was column chromatographed on silica gel, eluted with n-hexane:dichloromethane:ethyl acetate (5:4:1), to give six subfractions (II9-1-II9-6). subfraction II9-3 (62.2 mg) was further subjected to column chromatography over silica gel using a gradient mixture of n-hexane-dichloromethane-ethyl acetate (7:2:1) to give 1 (12.2 mg) and 2 (8.3 mg) .
General Experimental Procedure
Melting points were determined on an electrothermal melting point apparatus. The IR spectra were recorded on a Perkin-Elmer spectrum-100 FT-IR in KBr. Mass spectra were obtained with a Synapt G2 mass spectrometer instrument. NMR data were recorded on a JEOL-ECZ-600 spectrometer at
600 MHz for 1H and 150 MHz for 13C. Chemical shifts are given on a δ (ppm) scale with tetramethylsilane (TMS) as an internal standard. Column chromatography was conducted on silica gel 60 (Merck). TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% H2SO4 in ethanol, followed by heating.
RESULTS AND DISCUSSION
Cabraleadiol (1)
Physical properties: colourless needle crystal, m.p. 170-172oC. IR λmax (KBr) cm-1: 3457, 2948, 2861, 1461, 1380. HR-TOFI-MS m/z: 461.3879 [M+H]+. 1H-NMR (600 MHz, CDCl3) and 13C-NMR (125 MHz, CDCl3) see Table 1.
Cabraleahydroxylactone (2)
Physical properties: white amorphous powder, m.p. 240-242 oC, IR λmax (KBr) cm-1: 3550, 2900, 2810, 1760, 1457, 1386, 1249, 1196. HR-TOFI-MS m/z: 416 [M+H]+; 1H-NMR (600 MHz, CDCl3) and 13C-NMR (125 MHz, CDCl3) see Table 1.
Compound 1 was isolated as a colourless needle crystal. The molecular formula of 1 was determined to be C30H52O3 from its molecular ion peak [M+H]+ at m/z 460.3916 (calcd. for C30H52O3, 460.3920) in the LC-TOFMS. The IR spectrum showed absorption peaks due to hydroxyl (3457 cm-1), C-C aliphatic (2948 and 2861 cm-1 ) and ether functional groups (1380 cm-1).The 1H NMR spectrum (Table 1) displayed eight tertiary methyl singlets [δH0.87, 0.82, 0.92, 1.09, 1.17, 1.13, 0.95 and 0.84 (each 3H, s)], two oxymethine protons [δH 3.38 (1H, t, J=3.0 Hz, H-3 and 3.62 (1H, dd, J=4.8, 10.2 Hz, H-24)], and some aliphatic protons in the up field region. The13CNMR and DEPT-135 spectra (Table 1), exhibited the presence of eight methyl signals [δC 15.6 (Me-30), 22.2 (Me-29), 28.4 (Me-28), 24.1 (Me-27), 27.9 (Me-26), 27.3 (Me-21), 16.6 (Me-18) and 16.2 (Me-19)], ten methylenes [δC 33.7 (C-1), 25.4 (C-2), 18.3 (C-6), 34.8 (C-7), 21.7 (C-11), 27.1 (C-12), 31.5 (C-15), 25.9 (C-16), 35.3 (C-22), 26.4 (C-23)], six methines including two oxygenated methines [δC 76.4 (C-3), 49.6 (C-5), 50.7 (C-9), 42.8 (C-13), 49.8 (C-17), and
86.3 (C-24)] and eight quartenary carbon signals [δC 37.3 (C-4), 40.7 (C-8), 37.7 (C-10) and 50.2 (C-14). The NMR data suggested that 1 had a triterpenoid tetracyclic skeleton similar to the dammarane-type triterpenoid (Harneti et al., 2014). The structure of the tetracyclic system in 1 was determined by analysis of COSY and HMBC spectra (Figure 2). Key HMBC spectra were the 2J correlations from the eight methyl groups (Me-18, Me-19, Me-21, Me-14, Me-26, Me-27, Me-29 and Me-30) to their attached carbons enabled the assignment of the eight singlet methyls. A secondary alcohol was assigned at C-3 by the HMBC correlations from H-1 (δH 1.42), H-2 (δH 1.55), and H-5 (δH 1.24) to C-3 (δC 76.4), whereas a tertiary alcohol was located at C-25 by the HMBC correlation from Me-26 (δH 1.17), Me-27 (δH 1.09), H-24 (δH 3.62) to C-25 (δC 70.3).
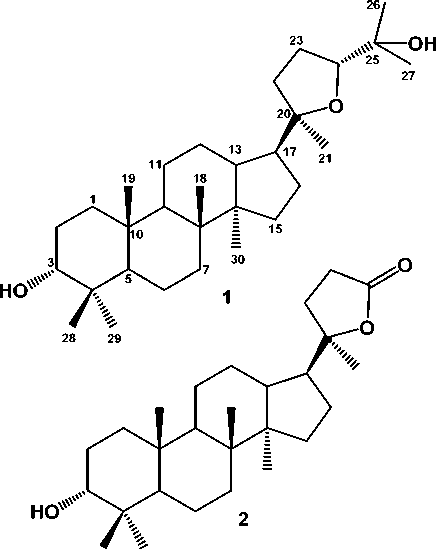
Figure 1. Chemical Structure of 1 and 2
The relative stereochemistry of 1 was identified based on coupling constants in the 1H-NMR and biogenetic point of view occurrence of dammarane-type triterpenoid in Chisocheton genus (Supratman et al., 2019).
In comparison of 1 with literature data of a cabraleadiol (Phongmaykin et al., 2008), showed good agreement, therefore compound 1 was identified as a cabraleadiol, which shown in this plant for the first time.
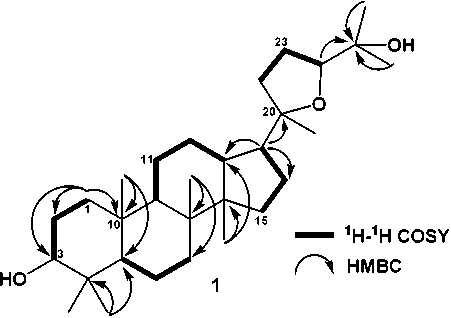
Figure 2. 1H-1H COSY and HMBC for 1
Compound 2 was obtained as a white amorphous powder. The IR spectra showed absorption peaks at 3550 cm-1 (OH), 2960 and 2865 cm-1 (aliphatic), 1382 and 1238 cm-1 (gem-dimethyl groups), and 1040 cm-1 (C-O). The NMR spectra of 2 was very similar with 1. The main differences was the absence of an isopropyl alcohol group at [(δH 1.17 (3H, s) and 1.09 (3H, s), δC 24.1 (Me-27), 27.9 (Me-26) and 70.3 (H-25)] and the presence of carbonyl lactone signal at δC 176.9, suggested that compound 2 was a lactone derivative of 1. In the HMBC spectrum, methylene signal at δH 2.52 was correlated to carbonyl lactone at δC 176.9, indicated that a carbonyl lactone was located at C-24 to make a lactone ring as a side chain ring.
The relative stereochemistry of 2 was identified based on coupling constants in the 1H-NMR and biogenetic point of view occurrence of dammarane-type triterpenoid in Chisocheton genus (Supratman et al., 2019). In comparison of 1 with literature data of a cabraleahydroxylactone (Phongmaykin et al., 2008), showed good agreement, therefore compound 1 was identified as a cabraleahydroxylactone, which shown in this plant for the first time.
Tabel 1. NMR data for compounds 1 and 2 (CDCl3 600 MHz for 1H and 150 MHz for 13C)
|
Position of C |
Compound 1 |
Compound 2 | ||
|
13C NMR δc (mult.) |
1H NMR δH (Integral, mult., J=Hz) |
13C NMR δc (mult.) |
1H NMR δH (Integral, mult., J=Hz) | |
|
1 |
33.7 (t) |
1.42 (1H, m) |
35.2 (t) |
1.17 (1H, m) |
|
1.34 (1H, m) |
1.50 (1H, m) | |||
|
2 |
25.4 (t) |
1.55 (1H, m) |
33.7 (t) |
1.40 (1H, dd; 2,4; 9,6) |
|
1.28 (1H, m) |
1.46 (1H, m) | |||
|
3 |
76.4 (d) |
3.38 (1H, t, 3) |
76.3 (d) |
3.37 (1H, s) |
|
4 |
37.3 (s) |
- |
37.3 (s) |
- |
|
5 |
49.6 (d) |
1.24 (1H, m) |
49.4 (d) |
1.95 (1H, m) |
|
6 |
18.3 (t) |
1.39 (1H, m) |
18.3 (t) |
1.37 (1H, m) |
|
1.30 (1H, m) |
1.56 (1H, m) | |||
|
7 |
34.8 (t) |
1.63 (1H, m) |
26.9 (t) |
1.71 (1H, m) |
|
1.76 (1H, m) |
1.82 (1H, m) | |||
|
8 |
40.7 (s) |
- |
40.6 (s) |
- |
|
9 |
50.7 (d) |
1.44 (1H, m) |
50.4 (d) |
1.41 (1H, dd, 2.4, 13.2) |
|
10 |
37.7 (s) |
- |
37.7 (s) |
- |
|
11 |
21.7 (t) |
1.53 (1H, m) |
25.4 (t) |
1.20 (1H, m) |
|
1.46 (1H, m) |
1.68 (1H, m) | |||
|
12 |
27.1 (t) |
1.75 (1H, m) |
21.3 (t) |
1.49 (1H, m) |
|
1.60 (1H, m) |
1.24 (1H, m) | |||
|
13 |
42.8 (d) |
1.62 (1H, m) |
43.2 (d) |
1.53 (1H, m) |
|
14 |
50.2 (s) |
- |
50.3 (s) |
- |
|
15 |
31.5 (t) |
1.04 (1H, m) |
31.2 (t) |
1.90 (1H, m) |
|
1.80 (1H, m) |
1.10 (1H, m) | |||
|
16 |
25.9 (t) |
1.51 (1H, m) |
25.1 (t) |
1.52 (1H, m) |
|
1.72 (1H, m) |
1.80 (1H, m) | |||
|
17 |
49.8 (d) |
1.83 (1H, m) |
49.5 (d) |
1.23 (1H, m) |
|
18 |
16.2 (q) |
0.95 (3H, s) |
15.6 (q) |
0.92 (3H, s) |
|
19 |
16.6 (q) |
0.84 (3H, s) |
16.1 (q) |
0.82 (3H, s) |
|
20 |
86.7 (s) |
- |
90.3 (s) |
- |
|
21 |
27.3 (q) |
1.13 (3H, s) |
25.4 (q) |
1.33 (3H, s) |
|
22 |
35.3 (t) |
1.22 (1H, m) |
31.3 (t) |
1.47 (1H, m) |
|
1.40 (1H, m) |
2.01 (1H, m) | |||
|
23 |
26.4 (t) |
1.85 (1H, m) |
29.3 (t) |
2.52 (1H, d, 10) |
|
1.90 (1H, m) |
2.62 (1H, d, 9.9) | |||
|
24 |
86.3 (d) |
3.62 (1H, dd, 4.8, 10.2) |
176.9 (s) |
- |
|
25 |
70.3 (s) |
- |
- | |
|
26 |
27.9 (q) |
1.17 (3H, s) |
- | |
|
27 |
24.1 (q) |
1.09 (3H, s) |
- | |
|
28 |
28.4 (q) |
0.92 (3H, s) |
28.4 (q) |
0.91 (3H, s) |
|
29 |
22.2 (q) |
0.82 (3H, s) |
22.2 (q) |
0.81 (3H, s) |
|
30 |
15.6 (q) |
0.87 (3H, s) |
16.4 (q) |
0.87 (3H, s) |
CONCLUSIONS
Two dammarane-type triterpenoids have been isolated from the the stembark of Chisocheton pentandrus belong to Meliaceae family and identified by spectroscopic data as cabraleadiol (1) and cabraleahydroxylactone (2). The investigation of these dammarane-type triterpenoids were shown in this species
for the first time and strenghten the occurance of this compounds in this genera.
ACKNOWLEDGMENTS
We thank Mr. Tomoki Nakamura in the Laboratory of Natural Products Chemistry, Faculty of Agriculture, Yamagata University, Japan for NMR measurements.
REFERENCES
Farabi, K., Harneti, D., Nurlelasari, Rani Maharani, R., Hidayat, A.T., Awang, K., Supratman, U., Shiono, Y. 2017. New cytotoxic protolimonoids from the stem bark of Aglaia argentea (Meliaceae). Phytochem. Lett. 21: 211215.
Fu, J., Wang, S., Lu, H., Ma, J., Ke, X., Liu, T., Luo, Y. 2015. In vitro inhibitory effects of triterpenoids from Chlorantus multistachys on epithelial-mesenchymal transition via dow-regulation of Runx2 activation in human breast cancer. Phytomedicine. 22: 165-172.
Harneti, D., Tjokronegoro, R., Safari, A., Supratman, U., Loong, X.M., Mukhtar, M.R., Mohamad, K., Awang, K., and Hayashi, H. 2012, Cytotoxic triterpenoids from the bark of Aglaia smithii., Phytochem. Lett. 5: 496–499.
Harneti, D., Supriadin, A., Ulfah, M., Safari, A., Supratman, U., Awang, K., Hayashi, H., 2014. Cytotoxic
constituents from the bark of Aglaia eximia (Meliaceae). Phytochem. Lett. 8: 28–31.
Heyne, K., 1982. The Useful Indonesian Plants, Research and Development Agency, Ministry of Forestry, Jakarta, Indonesia. 989-1012.
Katja, D.G., Farabi, K., Nurlelasari., Harneti, D., Mayanti, T., Supratman, U., Awang, K., Hayashi, H. 2017. Cytototoxic constituents from the bark of Chisocheton cumingianus (Meliaceae). J. Asian Nat. Prod. Res. 6:1–5.
Katja, D.G., Farabi, K., Nurlelasari, Hidayat, A.T., Mayanti, T., Harneti, D., Supratman, U.,
2016. A new 30-nor Trijugin-type Limonoid, Chisotrijugin, from the bark
of Chisocheton cumingianus (Meliaceae). Int. J. Chem. 8:30–34.
Nguyen, V.T., Tung, N.T., Cuong, T.D., Hung, T.M., Kim, J.A., Woo, M.H., Choi, J.S., Lee, J.H., Min, B.S. 2015. Cytotoxic and anti-angiogenic effects of lanostane triterpenoids from Ganoderma lucidum. Phytochem. Lett. 12: 69-74.
Phongmaykin, J., Kumamoto, T., Ishikawa, T., Suttisri, R., and Saifah, E. 2008. A New Sesquiterpene and Other Terpenoid Constituents of Chisocheton penduliflorus. Arch. Pharm. Res. 31(1): 21-27.
Supratman, U., Naibaho, W., Salam, S., Maharani, R., Hidayat, A.T., Harneti, D., Nurlelasari, Shiono, Y. 2019. Cytotoxic Triterpenoids from the Bark of Chisocheton patens Blume (Meliaceae). Phytochem. Lett. 30: 81– 87.
Supriatno., Nurlelasari., Herlina, T., Harneti, D., Maharani, R., Hidayat, A.T., Mayanti,T.,Supratman, U., Azmi, M.N., Shiono, Y., 2018. A new limonoid from stem bark ofChisocheton pentandrus (Meliaceae). Nat. Prod. Res. 25: 1–7.
Tian, Z., Yang, M., Huang, F., Li, K., Si, J., Shi, L., Chen, S., Xiao, P., 2005. Cytotoxicity of three cycloartane triterpenoids from Cimicifuga dahurica. Cancer Lett. 226:65-75.
Zhang, Y., Dewitt, D.L., Murugesan, S., Nair, M.G. 2005. Cyclooxigenase-2 enzyme inhibitory triterpenoids from Picrorhiza kurroa seeds. Life Sci.77: 3222-3230.
Yang, M.H., Wang, J.S., Luo, J.G., Wang, X.B., Kong, L.Y. 2012. Four
triterpenoids from Chisocheton
paniculates and their anti-inflammatory activities. Can. J. Chem. 90: 199-204.
93
Discussion and feedback