SYNTHESIS AND CHARACTERIZATION OF PHOTOCATALYS Fe2O3 PILLARED MONTMORILLONITE DOPED TiO2 AND ITS APPLICATION FOR RHODAMINE B PHODODEGRADATION USING VISIBLE LIGHT IRRADIATION
on
JURNAL KIMIA (JOURNAL OF CHEMISTRY) 14 (1), JANUARI 2020 DOI: https://doi.org/10.24843/JCHEM.2020.v14.i01.p14
p-ISSN 1907-9850
e-ISSN 2599-2740
SYNTHESIS AND CHARACTERIZATION OF PHOTOCATALYS Fe2O3 PILLARED MONTMORILLONITE DOPED TiO2 AND ITS APPLICATION FOR RHODAMINE B PHOTODEGRADATION USING VISIBLE LIGHT IRRADIATION
D. A. D. N. Dewi*, I N. Simpen, I W. Suarsa
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Udayana University, Jimbaran, Bali, Indonesia
*Email: natadev014@gmail.com
ABSTRAK
Lempung montmorillonit yang dimodifikasi dengan logam semikonduktor dapat berperan sebagai material fotokatalis. Pemilihan lempung montmorillonit didasarkan pada sifatnya yang mudah dimodifikasi serta memiliki luas permukaan spesifik yang tinggi. Penelitian ini bertujuan untuk memodifikasi lempung montmorillonit menjadi material fotokatalis untuk degradasi rhodamin B. Lempung montmorillonit dapat dimodifikasi dengan pemilaran menggunakan Fe2O3 dan diembankan dengan TiO2 menjadi lempung montmorillonit terpilar Fe2O3 terembakan TiO2 (Fe2O3-PILC/TiO2). Modifikasi dimaksudkan untuk meningkatkan luas permukaan spesifik serta jumlah situs aktif fotokatalis sehingga meningkatkan kemampuan fotodegradasi. Karakterisasi yang dilakukan antara lain karakterisasi keberhasilan pembentukan pilar menggunakan X-ray Diffraction (XRD), karakterisasi luas permukaan spesifik dengan metode BET (Bruneau, Emmet, dan Teller), karakterisasi jumlah situs asam-basa permukaan dengan metode titrasi. Fotokatalis dengan karakter terbaik adalah Fe2O3-PILC/TiO2 1:3 dengan memiliki luas permukaan spesifik, jumlah situs asam dan basa berturut-turut 45,947 m2/g, 20,1736 x 1023 situs/g dan 19,0044 x 1023 situs/g. Hasil optimasi kondisi fotodegradasi rhodamin B optimum dengan sinar tampak pada pH 3 menggunakan 400 mg fotokatalis adalah 99,84%.
Kata kunci: fotokatalis, Fe2O3, lempung montmorillonit, TiO2, rhodamin B
ABSTRACT
A montmorillonite clay modified with semiconductor metal can act as a photocatalyst material. Montmorillonite clays were chosen because of their natural characteristics which are easily to be modified and have high specific surface area. This research aims to modify montmorillonite clay into photocatalyst material for rhodamine B degradation. The montmorillonite clay was intercalated using Fe2O3 to produce Fe2O3-pillared montmorillonite clay, then doped with TiO2 to form a photocatalyst material Fe2O3-PILC/TiO2. Modifications were intended to increase the specific surface area and number of active photocatalyst sites and thus increase the ability of photodegradation. The characterization carried out included characterizing the pillar formation using X-ray Diffraction (XRD), specific surface area by the BET method (Bruneau, Emmet, and Teller), a the number of surface acid-base sites by the titration method. Photocatalyst with the best character was Fe2O3-PILC/TiO2 1:3 with specific surface area, number of acid and base sites respectively 45,947 m2/g, 20,1736 x 1023 sites/g and 19,0044 x 1023 sites/g. The result of photodegradation at optimum condition with visible light at pH 3 using 400 mg photocatalyst was 99.84%.
Keywords: photocatalyst, Fe2O3, montmorillonite clay, TiO2, rhodamine B
INTRODUCTION
The montmorillonite clay is a material that has been used as adsorbent. The popularity of montmorillonite material increases along the development of photodegradation methods in sewage treatment systems. Clay material is used as supported material for semiconductor photocatalysts. Montmorillonite clay was
selected because of its natural characteristics which are easily modified and has high specific surface area. The structure of montmorillonite clay material consists of two layers, the alumina silicate layer and the interlayer with non-strongly attached cations such as Na+, K+, and Ca2+ (Wijaya et al., 2002). The montmorillonite clay-layered structure allows the material to have cation
exchangeable property and has swelling ability. As result, montmorillonite clay is able to provide a place for certain molecular ions in the interlayer that can be used for clay pillarization (Utracki, 2004). Pillarization of montmorillonite clay is carried out by exchanging cations in its inter-layer with larger inorganic polycation. The presence of pillars will increase the basal spacing as well as the specific surface area of the clay. The pillarization process can be possibly done using intercalation method, followed by calcination process to form metal oxides which will take the roles as pillars (Gill et al., 2007).
Pillarization of montmorillonite clay with iron (III) oxide has been done by Widihati et al. (2004) which shown an increase in specific surface area from 95.0587 m2/g to 170.5416 m2/g at an optimum calcination temperature of 200oC. Iron (III) oxide is a semiconductor material that is widely used as photocatalyst because of its magnetic properties that not only guarantee an efficient process but also reusable (Bharati et al., 2009). Pillarization of montmorillonite clay using Fe2O3 can be carried out through an intercalation method using FeCl3 or Fe(NO3)3. The iron oxide (Fe2O3) is formed after thermal decomposition process or calcination (Greedon, 1994). The performance of the pillared clay photocatalyst can be optimized by doping another semiconductor material. Titanium dioxide (TiO2) in anatase phase is the most popular semiconductor material used as a photocatalyst. The presence of titanium oxide which doped into the Fe2O3 pillared clay is expected to increase the number of active sites along with the enhancement of the photodegradation performance.
Photodegradation methods are recently used in sewage treatment system, especially for textile wastewater. Textile wastewater treatment received a lot of attention due to its significant impact on environmental health, mainly related to the presence of synthetics dyes such as rhodamine B. Rhodamine B as a toxic non-biodegradable organic substance can be a threat to the aquatic biota and human health if being presence in water bodies (Forgacs et al., 2004). Photodegradation method which provides the alteration of pollutants by light into simpler and less harmful component is expected to be an alternative solution in overcoming the problem
of textile wastewater treatment (Fatimah et al., 2009).
MATERIALS AND METHODS
Materials
Montmorillonite clay, rhodamine B (C28H31N2O3Cl), FeCl3, NaOH, TiO2 anatase, NaCl, AgNO3, HCl, H2C2O4, phenolphthalein, ethanol 96%, aqua distillate, and aqua deionized.
Instruments
X-ray Diffraction (XRD) Phillips Analytical, Surface Area Analyzer (SAA) Quantachrome Autosorb iQ, and UV-Visible Spectrophotometer Shimadzu UV 1800.
Sample Preparation
The montmorillonite clay was saturated in 1M NaCl solution for 12 hours then washed until it was free of Cl ions (shown by negative test using 0.1 M AgNO3 solution). The saturated clay then dried at 110oC and sifted using a 100-mesh sieve.
Material Synthesis
The pillar solution consisting 0.1 M FeCl3 solution and 0.2 M NaOH solution using molar ratio of OH/Fe ≤ 2.0. The aging process was carried out at pH 1.8 for 24 hours. Solution obtained was mixed with clay samples (1 g clay: 45 mL solution) and stirred for 26 hours at room temperature. The suspension then filtered. Clay solids are washed using deionized water until free of Cl ions (shown by negative test against AgNO3 0.1M). The clay was dried at 110oC for 12 hours followed by calcination at 400oC for 4 hours (Hristodor et al., 2013). The calcinated montmorillonite then sifted using a 100-mesh sieve. The pillar formation was characterized using X-Ray diffraction (XRD) by analysing the 2θ shifting.
Solis state method was used to dope TiO2 into Fe2O3 pillared montmorillonite. Fe2O3 pillared montmorillonite mixed with TiO2 using several ratios which are 1:0; 1:1; 1:3; and 3:1. Each composition was added with 10 mL ethanol 96% then stirred with magnetic stirrer for 5 hours. The mixture was dried in an oven at 120oC for 5 hours, then sifted using 100-mesh sieve followed by calcination at 500oC for 5 hours. The specific surface area of each composition was analysed using the Bruneau-
Emmet-Teller (BET) method. The acid-base active sites were analysed using titration method.
Acid-Base Active Sites Characterization
Characterization of the acid-base active sites of photocatalyst Fe2O3 pillared montmorillonite/TiO2 was carried out quantitively using the acid-base titration method. The characterization of base site is done by adding 10.0 mL of 0.5M HCl solution into 500 mg photocatalyst. The suspension is stirred for 15 minutes then filtered. The filtrate was added 2-3 drops of phenolphthalein and titrated with 0.5M NaOH solution until the solution turn from colourless to pale pink. The volume of NaOH used in the titration was used to calculate the basicity of the photocatalyst surface using the following equation:
mmol HCln — mmol HCl1 Basicity = (1)
photocatalyst mass
(Kumar, et al., 1995)
The characterization of acid site is done by adding 10.0 mL of 0.5M NaOH solution into 500 mg photocatalyst. The suspension was stirred for 15 minutes then filtered. The filtrate was added 2-3 drops of phenolphthalein and titrated with 0.5M HCl solution until the solution turn from pink to colourless. The volume of HCl used in the titration was used to calculate the acidity of the photocatalyst surface using the following equation:
Acidity =
mmol NaOHn — mmol NaOH1
photocatalyst mass
(2)
(Kumar, et al., 1995)
According to the acid-base sites characterization and specific surface area analysis results, an optimum composition will be obtained. The optimum composition would be the one gives the highest number of active sites and surface area. This composition will be used to determine the optimum mass of photocatalyst for photodegradation of rhodamine B dye solution.
Photocatalyst’s Mass Optimization
The optimum composition of photocatalyst Fe2O3 pillared montmorillonite/TiO2 (100, 200, 300, 400, 500 mg) each added 25.0 mL of 100 mg/L rhodamine B dye solution. This mixture was
irradiated under visible light (30W HPL, 445 nm) while stirred using magnetic stirrer for 2 hours. The filtrate absorbance was measure with UV-Visible spectrophotometer using wavelength of 553.5 nm. The percentage of degradation (%D) of rhodamine B is calculated using the following equation:
%D =
[Λhβn] — [Λ⅛B1] [Λh!n]
×100% (3)
The percentage of degradation was used to determine the optimum mass of photocatalyst to degrade rhodamine B dye solution.
pH Optimization
A total 25.0 mL of 100 mg/L rhodamine B dye solution was added to the photocatalyst with optimum mass. The pH was adjusted to pH 2, 3, 4, 5, 6, 7, 8, and 9 by adding 0.5 M HCl or 0.5M NaOH to the solution. The mixture then irradiated for 120 minutes while stirred using magnetic stirrer. The absorbance of filtrate was measured with a UV-Visible spectrophotometer using wavelength of 553.5 nm. The percentage of degradation (%D) of rhodamine B is calculated using equation (3).
RESULT AND DISCUSSION
Analysis of Pillar Formation
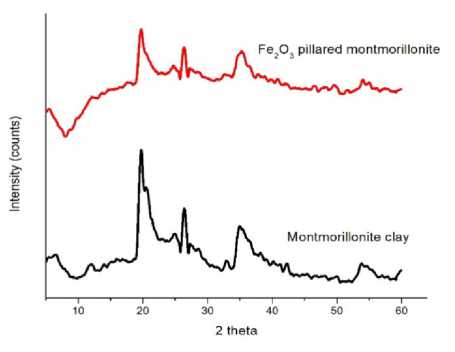
Figure 1. Diffractograms of montmorillonite clay and Fe2O3 pillared montmorillonite
Figure 1 shows XRD spectra of montmorillonite and Fe2O3 pillared montmorillonite clay recorded on Phillips diffractometer. The highest peak appears at 2θ=19.58° (d=4.54Å) indicates the presence of montmorillonite minerals (JCPDS, 29-1498). The peak that appears at 2θ=20.64° (d=4.30Å)
indicates the presence of quartz minerals, and peak at 2θ=26.33° (d=3.38Å) is a characteristic of illite (JCPDS, 5-0490). Figure 1 also shows that the pillarization process causes a general decrease in mineral intensity caused by the cation exchange between oligomer [Fe(OH)2]+n in pillar solution with mineral cations in montmorillonite clay (Cromain and Cahyaningrum, 2016).
The form of pillars was indicated by peak shifting at 2θ = 11.88° (d=7.45Å) on the lower diffraction angel, where the peak does not reappear on the pillared montmorillonite. The peak has shifted from 2θ = 11.88° (d=7.45Å) to 2θ < 11.88° (d>7.45Å) in the d001 because the formation of pillars has increased the basal spacing. These phenomena accordance with the Bragg’s law:
2d sin θ = n λ (4)
Bragg’s equation shows that if the basal spacing increased, the value of θ in the same n and λ condition will be decrease. The formation of pillar has also showed by the increased of specific surface area (Table 1).
Specific Surface Area
Specific surface area is one of the important characters that support the performance of photocatalysts. Specific surface area expresses the total photocatalyst surface area per photocatalyst mass (m2/g). The specific surface area of Fe2O3-PILC/TiO2 analysed by the BET Method using surface area analyser are shown in the Table 1.
Table 1. Specific Surface Area of Fe2O3-PILC/TiO2
|
Composite |
Specific surface area (m2/g) |
|
Montmorillonite clay* |
93.361 |
|
Fe2O3-PILC |
140.863 |
|
Fe2O3-PILC/TiO2 1 : 1 |
71.657 |
|
Fe2O3-PILC/TiO2 1 : 3 |
45.947 |
|
Fe2O3-PILC/TiO2 3 : 1 |
89.263 |
Source: Sitanggang, et al., (2017)
Photocatalyst Fe2O3-PILC showed the highest surface area at 140.863 m2/g. The specific surface area of Fe2O3 pillared montmorillonite has increased compares to montmorillonite clay. This is due to the increase of basal spacing which directly impacts the specific surface area. The presence of TiO2 material in the catalyst has cause a decrease in specific surface area. The specific surface area decreases along with the amount of TiO2 that doped into the Fe2O3 pillared montmorillonite. This depression is caused by trapped TiO2 molecules between the layers and on the surface area of pillared montmorillonite that it covers the pores (Wu et al., 2013). The lowest specific surface area is 45.947 m2/g and showed by Fe2O3-PILC/TiO2 1:3 respectively.
Specific surface area is not the only factor that support the performance of photocatalysts. The amount of acid-base active sites on photocatalyst surface also takes huge impact. Therefore, the result of active sites characterization must be considered.
Acid-Base Active Sites
The acidity and basicity of catalyst surface are values that represents the number of active sites of Bronsted or Lewis acid or base that is presence on the surface of the catalyst and it was expressed as the number (in millimoles) of acid or base in each g of catalyst. The number of active sites of acid and base catalyst surface can be determined by multiplying the acidity or basicity by the Avogadro’s number. The acidy-alkaline titration method was used to determine the acidity and basicity of the catalyst surface. The results of the acid-base active sites characterization are shown in the following table:
Table 2. Number of Acidic Active Sites of Photocatalyst Fe2O3-PILC/TiO2
|
Composites |
Acid active sites (sites/g) |
|
Fe2O3-PILC |
14.2775 x 1023 |
|
Fe2O3-PILC/TiO2 1 : 1 |
11.1199 x 1023 |
|
Fe2O3-PILC/TiO2 1 : 3 |
20.1736 x 1023 |
|
Fe2O3-PILC/TiO2 3 : 1 |
19.8456 x 1023 |
|
Table 3. Number |
of |
Base Active Sites of |
|
Photocatalyst Fe2O3-PILC/TiO2 | ||
|
Composites |
Base active sites (situs/g) | |
|
Fe2O3-PILC |
12.3491 x 1023 | |
|
Fe2O3-PILC/TiO2 1 : |
1 |
13.3847 x 1023 |
|
Fe2O3-PILC/TiO2 1 : |
3 |
19.0044 x 1023 |
|
Fe2O3-PILC/TiO2 3 : |
1 |
15.2657 x 1023 |
The acid-base active sites characterization of photocatalyst Fe2O3 pillared montmorillonite/TiO2 according to the Table 2 and Table 3 showed that Fe2O3 pillared montmorillonite/TiO2 1:3 is the composition with the highest acid-base sites. It has surface acidity, number of acid sites, basicity, and number of base sites of 3.3511±0.1159 mmol/g, 20.1736x1023 sites/g, 3.1569±0.1634 mmol/g and 19.0044x1023 sites/g respectively.
The active site plays an important role in the process of heterogeneous catalysis as well as the determination of reaction properties. The presence of active sites on the surface of the catalyst have two main functions, increasing reaction kinetics and improve product selectivity (Pan et al., 2018). The high number of active sites increased the formation of •OH radicals that play important roles in the process of photodegradation (Coleman et al., 2007). This condition means that the number of active acid-base sites on a photocatalyst are able to increase the performance of photocatalyst due to its function in degradation of rhodamine B. Therefore, the Fe2O3-PILC/TiO2 1:3 was chosen as the composition to be used in determining the optimum condition for rhodamine B photodegradation although its specific surface area is relatively lower than the other compositions.
Optimum Mass Condition
Optimum mass is the lowest photocatalyst mass needed to provide the highest percentage of degradation. The optimum mass of Fe2O3-PILC/TiO2 1:3 for rhodamine B photodegradation is shown by Figure 2.
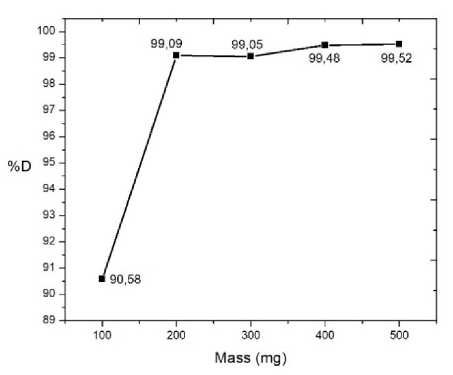
Figure 2. Plot between photocatalyst mass and percentage of degradation
The photocatalyst mass optimization showed an increase of %D from 90.58% to 99.09% along with the increase amount of photocatalyst used from 100 mg to 200 mg. The use of 300 mg photocatalyst showed a decrease in %D about 0.04% to 99.05%. Therefore the %D increase to 99.48% and 99.52% by using 400 and 500 mg photocatalyst respectively. Based on this data, 400 mg of photocatalyst was chosen as the optimum mass because of the %D has been relatively constant and has not decrease after this point.
Optimum pH Condition
The optimum pH is the pH condition of the solution needed to provide the highest %D in photodegradation of rhodamine B using Fe2O3-PILC/TiO2 1:3. The value of pH affect the surface charge and shifting the reaction potential which affect the degradation process. The relationship between the pH and %D in the rhodamine B photodegradation by Fe2O3-PILC/TiO2 photocatalyst is shown in Figure 3.
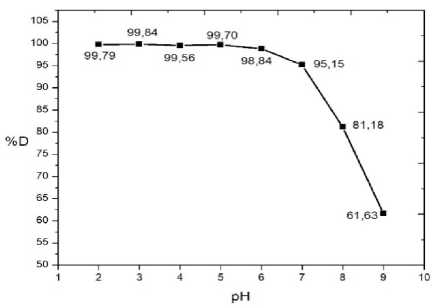
Figure 3. Plot between solution pH and %D
Figure 3 shows that rhodamine B is relatively easier to degrade in acidic pH than alkaline pH. Percentage of degradation decrease significantly from pH 6 until pH 9. Fauzi (2018) obtained a similar result with %D of rhodamine B of 97.40% occurred at pH 4. Sibarani (2016) also obtained the highest percentage of rhodamine B degradation of 95.05% at pH 4. These phenomena can be explained using surface charge theory. Titanium dioxide can be protonated or deprotonated under acidic or basic condition. The protonation and deprotonation reactions occur according to the following reaction:
Ti–OH + H+ → TiOH2+ Ti–OH + OH- → TiO- + H2O
The surface of TiO2 will be positively charged on an acidic medium and negatively charged on a alkaline medium. Mozia et al., (2009) reported that TiO2 has higher oxidation activity at low pH, but excess H+ ions can reduce the rate of reaction. TiO2 is a Lewis acid in acidic medium, so if pollutants are Lewis bases, it will be easily interacted with the photocatalyst. Rhodamine B forms negatively charged ions in water solvents. The difference surface charge between the positively charged photocatalyst and negatively charged rhodamine B pollutant causes electrostatic interaction and produces strong adsorption. Meanwhile, in alkaline solution there would be a repulsion force (Columbic repulsion) caused by the surface of the catalyst and pollutants that have same charged. According to this result, pH 3 was chosen as the optimum working pH for photodegradation of rhodamine B using Fe2O3-PILC/TiO2 1:3 with %D of 99.84%.
CONCLUSIONS
The montmorillonite photocatalyst with the best character was Fe2O3-PILC/TiO2 1:3 which had specific surface area, number of acid and base active sites of 45.947 m2/g, 200.1736 x1023 sites/g, and 19.0044 x 1023 sites/g respectively. The optimum condition for photodegradation of rhodamine B was at pH 3 using 400 mg of photocatalyst with percentage of degradation of 99.84%.
RECOMMENDATION
Further analysis of the solution that has been degraded needs to be done using LCMS or HPLC to ensure the product of the photodegradation process.
REFERENCES
Ali, R. and Siew, O.B. 2006. Photo degradation of New Methylen Blue N in Aqueous Solution Using Zinc Oxide and Titanium Dioxide as Catalyst. J. Teknologi. 45(3): 31-42.
Bharati, S., Nataraj, D., Mangalaraj, D., Masuda, Y., Senthil, K., Senthil, K., and Yong, K. 2009. Highly Mesoporus α-Fe2O3 Nanostructure: Preparation,
Characterization and Improved Photocatalytics Performance towards Rhodamine B (RhB. J. Phys. 43(1): 1-9.
Coleman H.M., Vimnoses V, Leslie G, and Amal R. 2007. Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J. Hazard. Mater. 146 (3): 496-501.
Cromain, C.A. dan Cahyaningrum, S.E. 2016. Karakterisasi Bentonit Terpilar Fe2O3 Sebagai Adsorben, UNESA Journal of Chemistry. 5(3): 48-53.
Fatimah, I., Shukla, P.R., and Kooli, F. 2009. Combined Photocatalytic and Fenton Oxidation of Methyl Orange Dye Using Iron Exchanged Titanium Pillared Montmorillonite. J. Appl. Sci. 9(20): 3725-3722.
Fauzi, W. A., Simpen, I N., dan Sudiarta, I W. 2018. Sintesis dan Karakterisasi Zeolit-TiO2 serta Pemanfaatannya sebagai Fotokatalis untuk Degradasi Rhodamin B. J. Kimia. 13(1): 74-81.
Forgacs, E. and Orus, G. 2004. Removal of Synthetic Dyes from Wastewater: a review. J. Env. Int. 30: 953-971.
Gill, A., Gandia, L.M., and Vicente, M.A. 2007. Recent Advances in the Synthesis and Catalitic Applications of Pillared Clays. Catal. Rev. Sci and Eng. 42(1&2): 145-212.
Greedon, J.E. 1994. Magnetic Oxides in Encyclopedia of Inorganic Chemistry Ed. R. Bruce King. John Wiley & Sons. New York.
Hristodor, C.M., Narcissa V., Rodica P., Violeta, E.P., Elena B., and Eveline Popovici. 2013. Preparation and Thermal stability of Al2O3-Clay and Fe2O3 Clay Nanocomposites, with Potential Application as Remediation of Radioactive Effluent. J Therm and Calorim. 111: 1227-1234.
Kumar, P., Jasra, R.V., and Bhat, T.S.G. 1995. Evolution of Porosity and Surface Acidity in Montmorillonite Clay on Acid Activation. Ind. Eng. Chem. Res. 34: 1440-1448.
Mozia S, Morawski A.W, Toyoda M, and Inagaki M. 2009. Application of anatasephase TiO2 for decomposition of azo dye in photocatalytic membrane reactor. J. Desalination. 241(1-3): 97-105.
Pan, Y., Shen, X., Yao, L., Bentalib, A., and Peng, Z. 2018. Active Sites in Heterogeneous Catalytic Reaction on Metal and Metal Oxide: Theory and Practice. J. Catalysts. 8:478.
Sibarani, J., Purba, D.L., Suprihatin, I.E., dan Manurung, M. 2016. Fotodegradasi Rhodamin B Menggunakan ZnO/UV/Reagen Fenton. J. Cakra Kimia, 4(1), 84-94.
Utracki, L.A. 2004. Clay-Containing Polymeric Nanocomposites. Vol. I. Rapra Technology. United Kingdom.
Widihati, I.A.G., Wijaya, K., dan Yahya, M.U. 2004. Sintesis Lempung Monmorillonit Terpilar Fe2O3 dan Kajian Sifat-Sifat Kimia Fisiknya. Thesis. Program Pasca Sarjana Universitas Gadjah Mada. Yogyakarta.
Wijaya, K., Pratiwi, A.S., Sudiono, S., dan Nurahmu, E. 2002. Study of Thermal and Acid Stability of Bentonite Clay. Indonesian J. Chem. 2 (1): 22-29.
Wu, H., Zhang, J., Wei, Q., Zheng, J. and Zhang, J. (2013). Transesterification of Soybean oil to Biodiesel Using Zeolit Supported CaO as Strong Base Catalysts. J. Fuel Processing Technology. 109: 1318.
88
Discussion and feedback