THE QUALITY OF COCONUT OIL PREPARED USING HEATING TECHNIQUE WITH ADDITION OF CARROT POWDER (Daucus carrota L) AS NATURAL ANTIOXIDANT
on
p-ISSN 1907-9850 e-ISSN 2599-2740
THE QUALITY OF COCONUT OIL PREPARED USING HEATING TECHNIQUE WITH ADDITION OF CARROT POWDER (Daucus carrota L) AS NATURAL ANTIOXIDANT
N. M. Suaniti*, M. Manurung, O. Ratnayani, and A. A. I. S. J. Dewi
Chemistry Program of Study, Faculty of Mathematics and Natural Sciences, Udayana University, Bukit Jimbaran, Bali, Indonesia
*Email: madesuaniti@unud.ac.id
ABSTRACT
Spoilage of coconut oil is indicated by rancidity caused by the oxidation and hydrolysis reactions. One of the efforts that can be carried out to inhibit the rancidity is by adding a natural antioxidant, such as carrot (Daucus carrota L) powder, into the coconut oil. This research aimed to find out the effect of the addition of carrot powder into the coconut oil on some parameters namely iodine number, peroxide number, FFA level, acid value and water content. The coconut oil was prepared by heating technique followed by the addition of carrot powder in the ratio of coconut oil: carrot of 100:1, 100:2, 100:3, 100:4, 100:5, and coconut oil : BHT (Butylated Hydroxy Toluene) of 100:1 as the positive control. The results were then compared to the Indonesian National Standard of SNI 01-2902-1992. It was found that the coconut oil added with carrot powder in the ratio of 100:4 showed the best quality with iodine number of 8.4092 ± 0.5761g iodine/100g, peroxide number of 3.2363 ± 1.9168mg O2/100g, Free Fatty Acid level (FFA) of 0.1676 ± 0.0037 %, acid value of 0.4656 ± 0.0119mg KOH/g and water content of 0.1038 ± 0.0068 %. The characterization using FTIR (Fourier-transform infrared spectroscopy) of such quality of coconut oil indicated some functional groups of OH, CH, CH3, C=O, C≡C aliphatic and C=C aliphatic were contained.
Keywords: carrot powder, coconut oil, natural antioxidant
ABSTRAK
Kerusakan minyak kelapa ditandai dengan ketengikan yang diakibatkan oleh terjadinya reaksi oksidasi dan hidrolisis. Salah satu upaya yang dilakukan untuk menghambat ketengikan tersebut adalah dengan penambahan antioksidan alami yaitu wortel (Daucus carrota L). Penelitian ini bertujuan untuk mengetahui pengaruh penambahan serbuk wortel ke dalam minyak kelapa terhadap bilangan iod, bilangan peroksida, kadar FFA, bilangan asam, dan kadar air. Pembuatan minyak kelapa dilakukan dengan teknik pemanasan dilanjutkan dengan penambahan serbuk wortel dengan perbandingan minyak kelapa:wortel 100:1, 100:2, 100:3, 100:4, 100:5, dan minyak kelapa:BHT(Butylated Hydroxy Toluene )100:1 sebagai kontrol positif. Hasil yang diperolehselanjutnyadibandingkan dengan SNI 01-2902-1992. Hasil penelitian menunjukkan bahwa minyak kelapa dengan penambahan serbuk wortel 100:4 memiliki kualitas terbaik yaitu bilangan iod 8,4092 ± 0,5761 g iod/ 100 g, bilangan peroksida 3,2363 ± 1,9168 mg O2/100g, kadar asam lemak bebas (FFA) 0,1676 ± 0,0037 %, bilangan asam 0,4656 ± 0,0119 mg KOH/ g dan kadar air 0,1038 ± 0,0068 %. Karakterisasi menggunakan FTIR (Fourier-transform infrared spectroscopy) terhadap minyak kelapa dengan kualitas terbaik tersebut menunjukkan adanya gugus fungsi OH, CH,CH3, C=O, C≡C alifatik dan C=C alifatik.
Kata kunci: antioksidan alami, minyak kelapa, serbuk wortel
INTRODUCTION
Nowadays, cooking oil as food processing material becomes a basic need for human beings. In Indonesia, coconut is one of the sources for making cooking oil. However, coconut oil can go rancid easily due to air or water contamination induced by oxidation and hydrolysis reactions (Sudarmadji, 1997). Contact between oxygen and fat or oil can cause the oxidation reaction, generating rancidity
because of the formation of aldehyde compounds (Kataren, 1986). The rancidity of cooking oil can be inhibited by adding antioxidants. Antioxidants are compounds that can slow down or inhibit oxidation reactions of the cooking oil (Pokorny et al., 2001).
There are many natural and synthetic antioxidants. Natural antioxidants are derived from extracts of natural products, while synthetic ones are made through chemical reactions such as Butylated Hydroxy Toluene
(BHT) and Butylated Hydroxy Anisol (BHA) (Buck, 1991).BHT and BHA can have negative impacts on human health such as liver and lung disfungtions and toxication. Therefore, it is necessary to have natural antioxidants as alternatives. Vegetables and fruits are the source of natural antioxidants, such as carrots (Daucus carrota L) which contain ascorbic acid, tocopherol and betacarotene (Kumalaningsih, 2006).
Utilization of carrots in the preparation of pure coconut oil or virgin coconut oil (VCO) had been carried out by Makalalag (2010) and Momuat et al. (2011). Makalalag explained that pure coconut oil could be made from fresh coconut without heating. Pure coconut oil produced was orange color. Momuat et al. showed that the addition of carrots in the virgin coconut oil could inhibit the oxidation reaction and thus the oil quality was better than pure coconut oil without the addition of carrots. Moreover, the addition of carrots in the home made cooking oil was also conducted by Almunady (2011) which indicated that carrot powder as antioxidant was able to inhibit the increase of peroxide numbers, but it was less effective than using the synthetic BHT antioxidant.
Based on this background, study on the making of coconut oil with heating technique was carried out, and then dry carrot powder (Daucus carrota L) was added into the oil. The benefits of using carrots are because they are natural antioxidants and can provide attractive color as well as increase nutritional value where carotenoids are precursors of vitamin A. The synthetic antioxidant BHT was used as positive control. Furthermore, the quality of coconut oil was determined through the iodine number, peroxide number, FFA level, acid value and water content, for certain storage time, to meet the Indonesian National Standard for coconut oil (SNI 01-2902-1992).
MATERIALS AND METHOD
Materials
In this study, carrots were taken from the Ubud Traditional Market, Ubud District and coconuts from Payangan Village Payangan District, Gianyar Regency, Bali Indonesia.
The chemicals used in this research were water, distilled water, Butylated Hydroxy Toluene (BHT), chloroform, Hanus reagent, 15% KI solution, saturated KI solution, 0.1 N Na2S2O3 solution, glacial acetic acid, 1% starch, 95% alcohol, H2C2O4, K2Cr2O7, phenolphthalein (PP) indicator and 0.1 N KOH.
The chemical apparatus applied were oven, sieves, blender, separating funnels, burettes, statives, clamps, Erlenmeyer flasks, measuring cups, beaker cups, volume pipettes, pipettes, funnels, filter paper, gauze, water bath, clear glass bottles, frying pan, stirrer, grater machine, fillers, knife, plastic jars and FTIR SHIMADZU Prestige 21 Spectrophotometer. The research was conducted at the Research Laboratory, Chemistry Program of Study of the Faculty of Mathematics and Natural Sciences, Udayana University.
Method
Preparation of carrot powder
Preparation of carrot powder was carried out by washing the carrots until clean, cut thinly and boiled for 1-3 minutes, then dried in an oven at 55-65oC for 8-10 hours. After being dried they were crushed using a blender and sieved smoothly (80 mesh), then wrapped and stored in a closed container (Almunady, 2011).
Preparation of coconut oil
About 20 kg of grated coconut added with water in a ratio of 1: 2 was squeezed then filtered. The resulting coconut milk was placed in a transparent plastic container, allowed to stand for 3 hours. The coconut milk then would separate into three layers: the top layer in the form of cream (oil-rich), the middle layer contains water or skim (protein-rich) and the bottom layer in the form of sediment. The cream on the top layer called blondo was then placed on a pan, and heated at a temperature of 80oC while being stirred continuously until there was a separation of the blondo with the oil.
The oil obtained was the crude oil. To get higher quality oil, the oil was heated again at a temperature of 60oC to produce clear oil as well as reduce the water content. The oil was then cooled and filtered with a filter paper, and thus the coconut oil was ready for use and the yield of the oil was calculated.
Addition of carrot powder into the coconut oil
A total of 5 of 250 mL Erlenmeyers each filled with 100 g of coconut oil then added with carrot powder 1g, 2g, 3g, 4g, and 5g respectively. As a comparison, 100 g of coconut oil was prepared without any addition of carrot powder and also 100 g of coconut oil with the addition of 1g BHT. Then the samples were stored in the covered glass bottles for 30 days at room temperature. Every week the testing was done for iodine numbers, peroxide numbers, FFA levels, acid values and water content. At the end of the test, the sample with the best quality was analyzed using FTIR SHIMADZU Prestige 21 Spectrophotometer.
Analysis of the quality of coconut oil
1. Determination of iodine number
A total of 2.50 g of sample was put into a closed Erlenmeyer, and then 5 mL of chloroform and 12.5 mL of Hanus reagent were added. The sample was left in the dark room for 30 minutes while being shaken occasionally. After that, the sample was added with 5 mL of 15% KI solution and 50 mL of boiled distilled water, then immediately titrated with Na2S2O30.1 N until the solution turned into light yellow color. It was then added with 2-3 drops of starch indicator and titrated with Na2S2O3 0.1 N until the blue color disappeared. This work was repeated three times(Jacobs, 1973).
A total of 2.50 g sample was placed in a closed 250mL Erlenmeyer flask, added with 25 mL of a glacial acetic acid -chloroform solution with a ratio of 3: 2 (v/v). After the oil was dissolved, 0.5 mL of saturated KI solution was added while being shaken then allowed to stand for 2 minutes, and finally added with 15 mL of distilled water.
After that, it was titrated with Na2S2O3 0.1N until the color became light brown. In order to know the end point of titration, 0.5 mL of 1% starch indicator was added and titrated with Na2S2O3 0.1N until the blue color disappeared. The titration was repeated three times (SNI 012902-1992).
A total of 2.50 g sample was put into a closed Erlenmeyer flask and 5 mL of 95% hot alcohol and 2 mL of phenolphthalein (PP) indicator were added and then titrated with a standardized 0.1 N KOH solution until turned into pink color and not disappeared within 30 seconds (Almunady, 2011).
Acid value is the amount of KOH (in milligrams) needed to neutralize 1 gram of sample. Acid values of coconut oil were calculated using the equation of (SNI 37412013):
Acid value (mg KOH/ g) =
56,1 × V × N W
A total of 2.50 g sample weighed with weighing bottle. It was then heated at a temperature of (130 ± 1) oC in the oven for 30 minutes and after that cooled in a desiccators for 30 minutes. The weighing bottle then weighed again. The heating and weighing of the sample repeated until constant weight was obtained (SNI 01-2902-1992).
RESULTS AND DISCUSSION
Preparation of carrot powder
Preparation of carrot powder was done by cutting the carrots thinly and boiling for 1-3 minutes which aimed to kill microbes as well as activate the antioxidants, and then dried in the oven at a temperature of 55-65oC for 8-10 hours. After that it was crushed with a blender and sieved smoothly (80 mesh). It produced as much as 54.73 g of carrot powder with yellow color and typical carrot smell. The powder was ready to be added into the coconut oil.
Preparation of coconut oil
Out of 28.80 kg of grated coconut from fresh coconuts obtained from Payangan Village, Gianyar Regency, as much as 2.98 kg of coconut oil with clear color and typical smell of coconut was produced with a yield of 10.37%.
Based on the research of Ruskandi (2004), the yield of traditional coconut oil heated with a temperature of 75oC was 11.47%.
Determination of iodine number
The iodine number was used to indicate the number of double bonds or unsaturated fatty acids that absorbed a number of iodine so that they formed a saturated bond (Sudarmaji et al., 1997). In this study, the test results of iodine number for 4 weeks were shown in Figure 1.
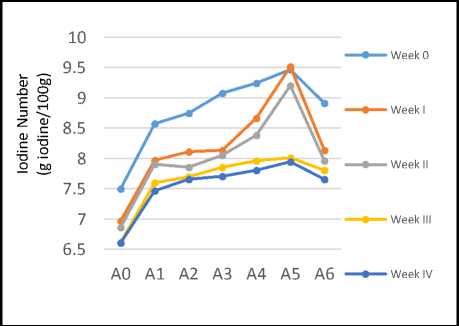
Figure1. Iodine number of coconut oil during 4 weeks (A0: without addition of carrot, A1-A5: with addition of carrot powder in the ratio of coconut oil:carrot of 100:1, 100:2, 100:3 100:4 and 100:5 respectively, A6: with addition of 1g of BHT per 100g oil)
Figure 1 showed that in general the addition of carrot powder into the coconut oil resulted in iodine numbers which had met the SNI 01-29021992(8-10mg iodine/100g) at the beginning of the test (week0), except for the coconut oil without the addition of carrot powder (A0). In the following weeks generally the iodine numbers tended to decrease but still met the national standard until week II, and only slightly declined out of the standard at week III and IV except for sample A4 and A5 that met the standard requirement until week III and IV respectively.
Thus, the addition of carrot powder into the coconut oil could increase the quality of the coconut oil, where sample A4 and A5 could maintain the quality of the oil longer than others.
Determination of peroxide number
Peroxide numbers> 5 indicate very poor oil quality, usually identified from the unpleasant odors (SNI 01-2902-1992). Peroxide number is
the most important value to determine the degree of damage of the oil or fat. The results of peroxide number assaying this research during for 4 weeks can be seen in the Figure 2.
The samples of coconut oil added with carrot powder A4 and A5 had the lowest peroxide number at 1.6656 mg O2/100g, so that the optimum composition for inhibiting oxidation reaction was the ratio of coconut oil: carrot of100:4 since less carrot powder was added yielded in the same peroxide number.
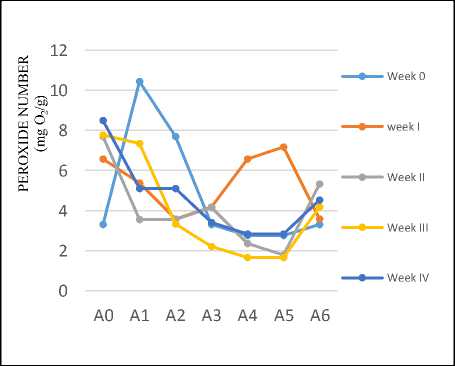
Figure2. Peroxide number of coconut oil during 4 weeks (A0: without addition of carrot, A1-A5: with addition of carrot powder in the ratio of coconut oil: carrot of 100:1, 100:2, 100:3 100:4 and 100:5 respectively, A6: with addition of 1g of BHT per 100g oil)
In general, the peroxide numbers of coconut oil with the addition of carrot powder were lower than coconut oil without the addition of carrot powder (A0) as well as with the addition of BHT 1g/ 100g coconut oil (A6). This indicated that carrots had antioxidant properties that could inhibit the oxidation reaction of the oil and could maintain the quality of the oil very well.
Determination of Free Fatty Acid (FFA) Level
Free fatty acid levels are determined from the hydrolysis process, which is the decomposition of fat or triglycerides by water molecules that produce glycerol and free fatty acids (Ketaren, 1986). In this study, the measurement results of the FFA level of the coconut oil with and without the addition of carrot powder is shown in Figure 3.
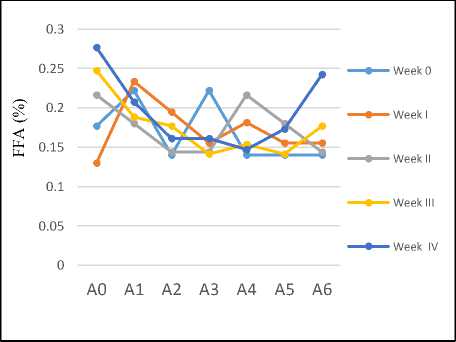
Figure 3. FFA level of coconut oil during 4 weeks (A0: without addition of carrot, A1-A5: with addition of carrot powder in the ratio of coconut oil: carrot of 100:1, 100:2, 100:3 100:4 and 100:5 respectively, A6: with addition of 1g of BHT per 100g oil)
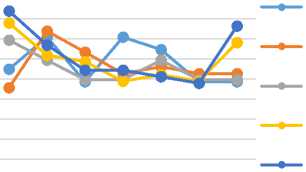
The results of measuring the acid values of the coconut oil in this research can be seen in Figure 4. It showed that the acid value of sample A0 declined at the first week then increased until week IV, while for sample A1 it inclined at the beginning but then decreased from the second week. For sample A2 there was an unstable change of the value every week while sample A3 and A4 tended to decrease. Sample A5 did not experience any significant changes every week. For oil with the addition of 1g BHT (A6) generally tended to increase. The acid value of cooking oil without the addition of carrot powder (A0) had the highest acid value of 0.7375mg KOH/g which exceeded the acid value based on SNI 3741- 2013.
The FFA level of sample A0 (without addition of carrot) decreased at the first week but then kept increasing in the following weeks. For sample A1, the FFA increased at the first week but then declined in the second week and afterwards, meanwhile sample A2 had unstable change of FFA every week. The FFA of sample A3 tended to incline at the third and fourth weeks while sample A4 tended to decrease from week III. The sample A5 did not experience significant changes on a weekly basis while A6 (with addition of synthetic antioxidant BHT) experienced a significant increase at the fourth week.
The results showed that generally the addition of carrot powder into the coconut oil did not cause a significant change in the level of free fatty acid and all of them had met the requirement of SNI 01-2902-1992 which is the maximum of 5%.
Determination of acid value
Acid values signified the amount of free fatty acids in oil expressed by mg base per 1gram of oil. The higher the acid number, the lower the oil quality (Haryani, 2006).
Q op U S
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
Week 0
Week I
Week II
Week III
Week IV
Figure 4. Acid value of coconut oil during 4 weeks (A0: without addition of carrot, A1-A5: with addition of carrot powder in the ratio of coconut oil: carrot of 100:1, 100:2, 100:3 100:4 and 100:5 respectively, A6: with addition of 1g of BHT per 100g oil)
A0 A1 A2 A3 A4 A5 A6
Determination of water content
Water content is the amount (percent %) of water contained in a material that evaporates when heated at a certain temperature and time. If there is water in the coconut oil, then it will result a hydrolysis reaction which can cause damage to the oil identified with the unpleasant rancid smell. The results of water content measurement in this study can be seen in Figure 5 below.
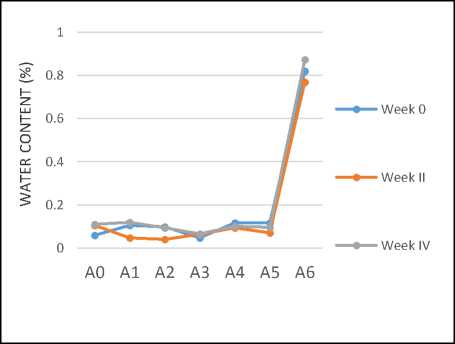
Figure 5. Water content of coconut oil during 4 weeks (A0: without addition of carrot, A1-A5: with addition of carrot powder in the ratio of coconut oil: carrot of 100:1, 100:2, 100:3 100:4 and 100:5 respectively, A6: with addition of 1g of BHT per 100g oil)
Figure 5 indicated that the water content of sample A0, A1, A2, A3, A4, and A5 did not experience significant changes on a weekly basis and generally had met the water content standard of SNI 01-2902-1992 which was the maximum of 0.5%. Thus, the addition of carrot powder into the coconut oil did not cause a large change of the water content. However, when 1g BHT was added into 100g coconut oil, it had the highest water content of 0.8736%.
Analysis of Functional Groups using FTIR
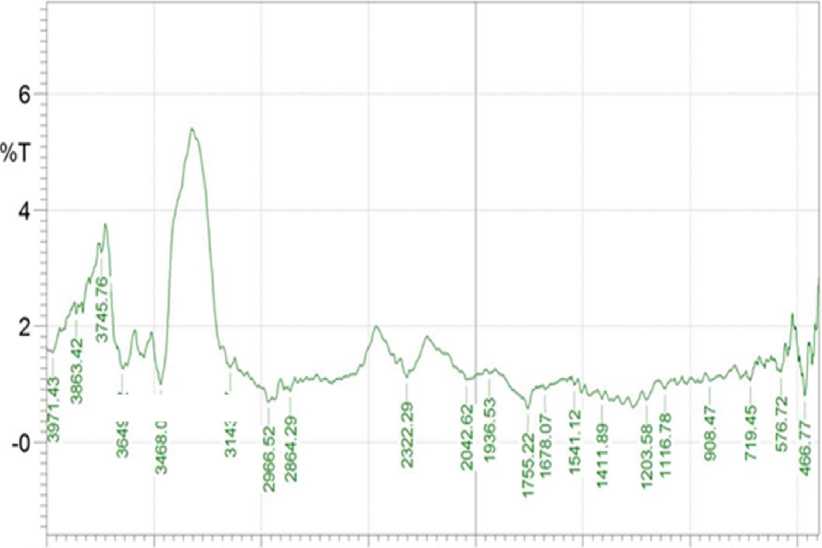
Figure 6 below showed the infrared spectra of coconut oil with addition of carrot powder in the ratio of coconut oil: carrot of 100:4.The infrared spectra data of coconut oil with addition of carrot powder in the ratio of coconut oil: carrot of 100:4 is presented in Table 1.
4000 3500 3000 2500 2000 1500 1000 500
Figure 6. FTIR spectra of coconut oil added with carrot powder in the ratio of coconut oil: carrot of 100:4
1/cm
Table 1. Infrared spectra of coconut oil with addition of carrot powder in the ratio of coconut oil: carrot of 100:4
|
Wave number (cm-1) |
Band |
Absorption Intensity |
Functional Group | |
|
Spectrum |
Reference | |||
|
3649,32 |
3700-3500 |
Sharp |
Medium |
Free -OH (stretching) |
|
3468,01; 3143,97 |
3500-3300 |
Sharp |
Medium |
Aliphatic -CH(stretching) |
|
2966,52 |
2960-2870 |
Sharp |
Strong |
Aliphatic-CH (-CH3stretching) |
|
2322,29; 2042,62 |
2400-2000 |
Sharp |
Medium |
Aliphatic-C≡C- (stretching) |
|
1755,22; 1678,07 |
1820-1600 |
Sharp |
Strong |
-C=O Carbonyl (stretching) |
|
1541,12 |
1650-1500 |
Sharp |
Weak |
Aliphatic-C=C- (stretching) |
|
1411,89 |
1500-1400 |
Sharp |
Strong |
Aliphatic-CH (bending) |
|
1203,58 |
1300-1000 |
Sharp |
Strong |
-C-O Alcohol (stretching) C-C (bending) |
|
576,72; 466,77 |
675-400 |
Sharp |
Strong |
Out of plane -CH (bending) |
The FTIR results showed that the absorption in the wave number area of 3649.32 cm-1indicated the vibration of the free OH group. This estimation was strengthened by the appearance of absorption in the wave number area of 1203.58cm-1 which was the absorption of C-O alcohol. Absorption in the wave number area of 2966.52 cm-1 was caused by the vibration of the CH3 group which was strengthened by the appearance of absorption at the bending area of wave number 1411.89 cm-1. Meanwhile, the absorption in the wave number region of 2322.29 cm-1 and 2042.62 cm-1 was due to the vibration of the aliphatic C≡C group.
This estimation was confirmed by the appearance of absorption in the wave number area of 3468.01 cm-1 and 3143.97 cm-1 which were the absorption from CH aliphatic. Moreover, the absorption in the wave number region of 1755.22 cm-1was because of the vibration of the C=O group. The absorption band at the wave number area of 1541.12 cm-1was assigned to be the absorption of=C aliphatic (Sastrohamidjojo, 1991; Silverstein et al., 1991).
Therefore, the FTIR results showed that coconut oil with addition of 4g carrot powder/100g oil contained functional groups of OH, CH, CH3, C=O, aliphatic C≡C and aliphatic C=C.
CONCLUSIONS
The quality of coconut oil prepared by heating technique with the addition of carrot powder (Daucus carrota L) had met the Indonesian National Standard of SNI 01-29021992.
The best quality of coconut oil was produced with the ratio of coconut oil and carrot powder of 100:4, with the iodine number of 8.4092 ± 0.5761g iodine/100g, peroxide number of 3.2363 ± 1.9168mg O2/100g, Free Fatty Acid level (FFA) of 0.1676 ± 0.0037 %, acid value of 0.4656 ± 0.0119mg KOH/g and water content of 0.1038 ± 0.0068 %.
REFFERENCES
Almunady, T. P., 2011, Pengaruh Penambahan Tepung Wortel (Daucus carrota L.) Terhadap Bilangan Peroksida dan Asam Lemak Bebas pada Minyak Goreng Curah, Jurnal Penelitian Sains. 14 (2C): 14204-18 – 14204-21.
Buck, D.F., 1991, Antioxidant, didalam: J. Smith, editor. Food Additive User’s Handbook. Blackie Academic dan Professional, Glasgow-UK.
Haryani, K., Widayat., dan Suherman, 2006, Optimasi Proses Adsorbsi Minyak Goreng Bekas Dengan Adsorbent Zeolit alam: Studi Pengurangan Bilangan Asam. Jurnal Teknik Gelagar, 17(2), 77 – 82.
Jacobs, M. B., 1973, The Chemical Analysis of Food and Food Products, Roberte Krieger Publishing Co., Inc., New York.
Ketaren, S., 1986, Pengantar Teknologi Minyak dan Lemak Pangan, Universitas
Indonesia Press, Jakarta.
Kumalaningsih, S., 2006, Antioksidan Alami-Penangkal Radikal Bebas, Sumber, Manfaat, Cara Penyediaan dan Pengolahan., Trubus Agrisarana,
Surabaya.
Makalalag, E., 2010, Aktivitas Antioksidan
Ekstrak Wortel yang Ditambahkan
dalam Proses Pembuatan Minyak Kelapa Murni, Skripsi, FMIPA Universitas Sam Ratulangi, Manado.
Momuat, L. I., Meiske, S. S., dan Purwati, N. P., 2011, Pengaruh VCO Mengandung Ekstrak Wortel Terhadap Peroksida Lipid Plasma, Jurnal Ilmiah Sains, 11(2) : 296 – 301
Pokorny, N., and Yanishlieva, M. G., 2001, Antioxidants in Food, CRC Press, Boca Raton Boston New York Washington, DC.
Ruskandi dan Setiawan, O., 2004, Pembuatan Minyak Kelapa Secara Tradisional dengan Perlakuan Suhu Air yang Berbeda, Pusat Penelitian dan Pengembangan Peternakan, 12(3) : 202206.
Standar Nasional Indonesia (SNI), 1992, SNI 01-2902-1992,Mutu dan Cara Uji Minyak Kelapa, Badan Standarisasi Nasional, Jakarta.
Standar Nasional Indonesia (SNI), 2013, SNI 3741-2013 Minyak Goreng, Badan Standarisasi Nasional, Jakarta.
Sudarmadji, S., Suhardi., dan Haryono, B., 1997, Analisa Bahan Pangan dan Pertanian, PAU Pangan dan Gizi UGM, Yogyakarta.
124
Discussion and feedback