A TRITERPENOID COMPOUND FROM THE STEMBARK OF Aglaia argentea (MELIACEAE)
on
p-ISSN 1907-9850
e-ISSN 2599-2740
A TRITERPENOID COMPOUND FROM THE STEMBARK OF Aglaia argentea (MELIACEAE)
-
A. T. Hidayat1,2, K. Farabi1, M. Muhammad1, D. Harneti1, Nurlelasari1, R. Maharani1,2, K. Haikal2, U. Supratman1,2*, Y. Shiono3.
-
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Indonesia
-
2Central Laboratory of Universitas Padjadjaran, Jatinangor 45363, Sumedang, West Java, Indonesia 3Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan.
*E-mail : unang.supratman@unpad.ac.id
ABSTRAK
Senyawa triterpenoid, 3-epikabraleahidroksilakton (1) telah diisolasi dari ekstrak n-heksana kulit batang Aglaia argentea. Struktur kimia senyawa 1 diidentifikasikan berdasarkan data-data spektroskopi dan perbandingan data spektra yang diperoleh sebelumnya. Senyawa triterpenoid, 3-epikabrraleahidroksilakton (1) dilaporkan pada tumbuhan ini untuk pertama kali.
Kata kunci : Aglaia argentea, 3-epikabraleahidroksilakton, Triterpenoid, Meliaceae
ABSTRACT
A triterpenoid compound, 3-epicabraleahydroxylactone (1) has been isolated from the n-hexane extract of the stembark Aglaia argentea. The chemical structure of compound 1 was identified based on spectroscopic data and by comparison with the spectral data previously reported. A triterpenoid, 3-epicabraleahydroxylactone (1), was reported from this plant for the first time.
Keywords : Aglaia argentea, 3-epicabraleahydroxylactone, Triterpenoid, Meliaceae
INTRODUCTION
The genus Aglaia belong to Meliaceae family consists of approximately 120 species, which are mainly distributed in the tropical rainforest especially in the Southeast Asia and on the Pacific islands (Pannell, 1992). Previous phytochemical studies have revealed the presence of a variety of compounds with interesting biological activities, including rocaglamide (Su et al., 2006), protolimonoids (Farabi et al., 2017a), bisamides (Sianturi et al., 2015), dammaran-type triterpenoids (Harneti et al., 2012), cycloartane-type triterpenoids (Awang et al., 2012), glabretal-type triterpenoids (Su et al., 2006), steroids (Harneti et al., 2014; Farabi et al., 2017b) and flavonoids (Nugroho et al., 1999).
Triterpenoids are metabolites of isopentenyl pyrophosphate oligomers and represent the largest group of phytochemicals. It has been estimated that more than 20,000 triterpenoids exist in nature (Liby et al., 2007). They predominantly are found in various plants including sea-weeds as well as in wax-like coatings of various fruits and medicinal herbs, including apples, cranberries, figs, olives, mistletoe, lavender, oregano, rosemary and thyme (Rabi et al., 2009, Laszczyk, 2009; Ovensna et al., 2004; Neto, 2007; Gerhauser, 2008). Triterpenoids are biosynthesized in plants by the cyclization of squalene, a triterpene hydrocarbon and precursor of all steroids (Phillips et al., 2006). They can further be subclassified into diverse groups including cucurbitanes, cycloartanes, dammaranes,
euphanes, friedelanes, holostanes, hopanes, isomalabaricanes, lanostanes, limonoids, lupanes, oleananes, protostanes, sqalenes, tirucallanes, ursanes and miscellaneous compounds (Setzer et al., 2003; Petronelli et al., 2009; Mullauer et al., 2010). Although triterpenoid compounds of other Aglaia species have been investigated previously, but the triterpenoid compound of A. argentea is yet to be reported. We report herein the isolation and structure identification of triterpenoid compound, 3-epicabraleahydroxylactone (1) from the stembark of A. argentea.
MATERIAL AND METHODS
Plant Material
The stembark of A. argentea were collected in Bogor Botanical Garden, Bogor, West Java Province, Indonesia in January 2016. The plant was identified by the staff of the Bogoriense Herbarium, Research Center for Biology, Indonesian Institute of Science, Bogor, Indonesia and a voucher specimen (No. Bo-1288718) has been deposited at the herbarium.
General Experimental Procedure
Melting points were measured on an electrothermal melting point apparatus and are uncorrected. Added of UV Spectra were measured by using a TECAN Infinite M200 pro with MeOH. The IR spectra were recorded on a Perkin-Elmer spectrum-100 FT-IR in KBr. Mass spectra were obtained with a Synapt G2 mass spectrometer instrument. 1H, 13C, DEPT NMR spectral data and 1H-1H COSY, HMQC and HMBC experiments were performed on a JEOL ECZ-600 spectrometer at 600 MHz with CDCl3 as a solvent, chemical shifts are given on a δ (ppm) scale and tetramethylsilane (TMS) as an internal standard. Column chromatography was conducted on silica gel 60. TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% H2SO4 in EtOH, followed by heating.
Extraction and isolation
The crushed and dried bark (2.5 kg) of A. argentea was extracted with methanol (14 L) at room temperature for 5 days. The methanol extract was evaporated under reduce pressure to give a
brown residue (140 g g). The brown residue was first dissoved in H2O and then partitioned successively with n-hexane (3 L), EtOAc (3 L) and n-butanol (3 L). The n-hexane soluble fraction (14.1 g) was fractionated by vacuum liquid chromatography on silica gel using a gradient n-hexane-EtOAc to give ten fractions (A–J). Fraction D (340 mg) was column chromatographed on silica gel, eluted with n-hexane:EtOAc (1:1), to give six subfractions (D01–D06). Subfraction D05 was column chromatographed on octa desyl silane, eluted with MeOH:MeCN:H2O (6:1:3) to give 1 (20.6 mg).
3-epicabraleahydroxylactone (1). White crystals, m.p. (decomposed); IR (KBr) νmax 3477, 2942, 2890, 1715, 1471, 1387, 1075 cm-1; 1H NMR (CDCl3, 600 MHz); 13C NMR (CD3OD, 125 MHz), see Table 1. HRTOF-MS m/z 417.3105 [M+H]+, calcd. for C27H44O3 m/z 416.3290.
RESULTS AND DISCUSSION
The phytochemical test for the n-hexane extract showed the presence of triterpenoids. By using triterpenoid test to guide separations, the n-hexane fraction was separated by column chromatography over silica gel by gradient elution. The fractions were repeatedly subjected to normalphase column chromatography and preparative TLC on silica gel GF254 yielded a triterpenoud, 1 (Figure 1).
Compound 1, was obtained as white crystals. The molecular formula was established to be C27H44O3 based on The HR-TOFMS spectrum m/z 417.3105 [M+H]+, calcld. for C27H44O3 m/z 416.3290 together with NMR data (Tabel 1), thus requring six degrees of unsaturation. The UV spectrum showed no a conjugated double based on the absorption maximum above 20 nm. IR spectrum of 1 showed the presence of a hydroxyl group (3477 cm-1), aliphatic bands (2942 and 2860 cm-1), a carbonyl lactone (1715 cm-1), a gem-dimethyl (1471 and 1387 cm-1) and an ether (1075 cm-1).
1H-NMR spectrum of 1 showed the presence of six tertiary methyl resonances at δH 0.92 (3H, s, H-18), 0.82 (3H, s, H-19), 1.33 (3H, s, H-21), 0.91 (3H, s, H-28), 0.81 (3H, s, H-29) and 0.87 (3H, s, H-30), which characteristic signals for a nor-triterpenoid (Phongmaykin et al., 2008) and
an oxygenated methine at δH 3,37 (1H, s, H-3). 13C-NMR spectrum of 1 together with DEPT experiments showed the presence of twenty seven carbon signals consist of six methyls resonances at [δc 15.6 (C-18), 16.1 (C-19), 25.4 (C-21), 28.4 (C-28), 22.2 (C-29) and 16.4 (C-30)], an oxymethine signal at δc 76.3 (C-3), an oxygenated quartenary sp3 carbon at δc 90,3 (C-20) and an ester signal at δc 176.9 (C-24), indicated opening of C-25, C-26 and C-27 carbons and formed a lactone ring at C-24/20, supporting the presence of trisnortriterpenoid dammaran (Phongmaykin et al., 2008).
methyls at C-4 (2×), C-10, C-8, C-14 and C-20, respectively. Methylenes protons at δH 1.40 and 1.56 were correlated to an oxygenated carbon at δC 76.3 (C-3), whereas one of a gem-dimethyl was correlated to an oxygenated carbon at C-3 (δC 76.3), indicated that a secondary hydroxyl group was attached at C-3. A methylene protons at δH 2.52 and 2.62 were correlated to carbonyl lactone at δC 176.9 (C-24), whereas the methyl proton at δH 1.33 was correlated to oxygenated carbon at C-20 (δC 90.3) and C-17 (δC 49.5), indicated that a lactone ring was attached at C-17.
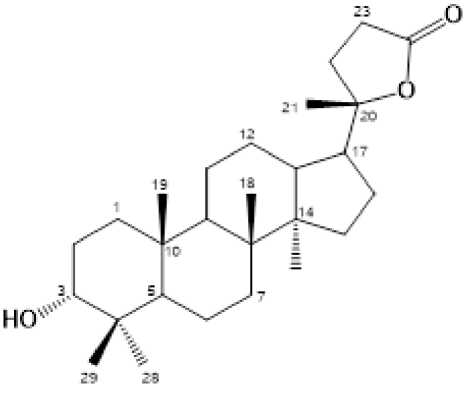
Figure 1. Chemical Structure for Compound 1
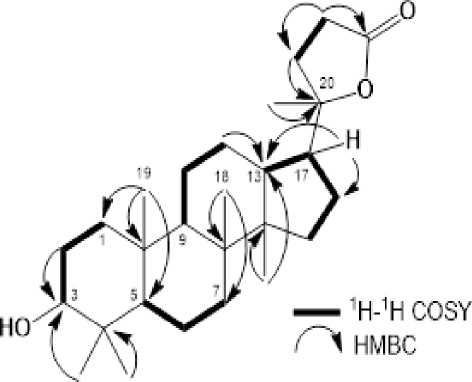
Figure 2. Selected 1H-1H COSY and HMBC correlations for 1
These functionalities accounted for one out of the total six degrees of unsaturation. The remaining five degrees of unsaturation were consistent with the trisnortriterpenoid dammaran with addition of a lactone ring.
In order to clarify the position of functional group in compound 1, 1H-1H COSY and HMBC experiments were carried out and the results was shown in Figure 2. The 1H-1H COSY spectrum of 1 showed correlations in H1-H2-H3, H5-H6-H7, H9-H11-H12-H13, H15-H16-H17, and H22-H23, supporting the presence of a tris-nortriterpenoid dammaran structure in 1. In the HMBC spectrum, the correlations arising from the tertiary methyl protons to their neighboring carbons enabled the assignment of the five singlet
A relative stereochemistry of a hydroxyl group at C-3 was determined on the basis of coupling constant, 3J 0 Hz in the 1H NMR and δC 90.3 (C-20) in the 13C NMR, indicating dan 3-OH as α-oriented, as well as a biogenetic point of view of the triterpenoid in the Aglaia genus. Consequently compound 1 was identified as a 3-epicabraleahydroxylactone, and was reported from this plant for the first time.
CONCLUSION
A trisnortriterpenoid dammaran, 3-epicabraleahydroxylactone (1) has been isolated from the n-hexane extract of the stembark Aglaia argentea. This investigation support the
occurances of triterpenoid compounds in the Aglaia genus.
Table 1. NMR Data for Compound 1 (500 MHz for 1H and 125 MHz for 13C in CDCl3)
|
Position of Carbon |
1H NMR δH (Integral., mult., J=Hz) |
13C NMR δC (mult.) |
|
1 |
1.17 (1H, m) |
35.2 (t) |
|
1.50 (1H, m) | ||
|
2 |
1.40 (1H, dd, 2.4, |
33.7 (t) |
|
9.6) | ||
|
1.56 (1H, m) | ||
|
3 |
3.37 (1H, m) |
76.3 (d) |
|
4 |
- |
37.3 (s) |
|
5 |
1.85 (1H, m) |
49.4 (d) |
|
6 |
1.37 (1H, m) |
18.3 (t) |
|
1.48 (1H, m) | ||
|
7 |
1.71 (1H, m) |
26.9 (t) |
|
1.84 (1H, m) | ||
|
8 |
- |
40.6 (s) |
|
9 |
1.41 (1H, dd, 2.4, |
50.4 (d) |
|
13.2) | ||
|
10 |
- |
37.7 (s) |
|
11 |
1.20 (1H, m) |
25.4 (t) |
|
1.34 (1H, m) | ||
|
12 |
1.49 (1H, m) |
21.3 (t) |
|
1.91 (1H, m) |
21.3 (t) | |
|
13 |
1.53 (1H, m) |
43.2 (d) |
|
14 |
- |
50.3 (s) |
|
15 |
1.10 (1H, m) |
31.2 (t) |
|
1.90 (1H, m) | ||
|
16 |
1.52 (1H, m) |
25.1 (t) |
|
1.42 (1H, m) | ||
|
17 |
1.23 (1H, m) |
49.5 (d) |
|
18 |
0.92 (3H, s) |
15.6 (q) |
|
19 |
0.82 (3H, s) |
16.1 (q) |
|
20 |
- |
90.3 (s) |
|
21 |
1.33 (3H, s) |
25.4 (q) |
|
22 |
1.47 (1H, m) |
31.3 (t) |
|
2.01 (1H, m) | ||
|
23 |
2.52 (1H, m) |
29.3 (t) |
|
2.62 (1H, m) | ||
|
24 |
- |
176.9 (s) |
|
28 |
0.91 (3H, s) |
28.4 (q) |
|
29 |
0.81 (3H, s) |
22.2 (q) |
|
30 |
0.87 (3H, s) |
16.4 (q) |
ACKNOWNLEDMENT
This investigation was financially supported by Directorate General of Higher Education, Ministry of Research, Technology and Higher Education, Indonesia (Postgraduate Grant, 2015206 by Unang Supratman).
REFERENCES
Awang, K., Loong, X., Leong, K. H., Supratman, U., Litaudon, M., Mukhtar, M. R., &
Mohamad, K. 2012. Triterpenes and steroids from the leaves of Aglaia exima (Meliaceae). Fitoterapia. 83, 1391–1395.
Farabi, K., Harneti, D., Nurlelasari., Maharani, R., Hidayat, A.T., Supratman, U., Awang K., and Shiono, Y. 2017a. Cytotoxic Steroids from the Bark of Aglaia argentea (Meliaceae). Chiang Mai University Journal of Natural Sciences (CMU-NS), 16(4), 293306.
Farabi, K., Harneti, D., Nurlelasari, Maharani, R., Hidayat, A,T., Awang, K., Supratman, U., Shiono, Y. 2017b. New cytotoxic protolimonoids from the stem bark of Aglaia argentea (Meliaceae). Phytochemistry Letters, 21, 211-215.
Gerhauser, C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Medica 2008, 74:1608– 1624.
Harneti, D., Tjokronegoro, R., Safari, A., Supratman, U., Loong, X., Mukhtar, M. R., Mohamad, K., Awang, K., & Hayashi, H. 2012. Cytotoxic triterpenoids from the bark of Aglaia smithii (Meliaceae).
Phytochemistry Letters. 5, 496–499.
Harneti, D., Supriadin, A., Ulfah, M., Safari, A., Supratman, U., Awang, K., & Hayashi, H. 2014. Cytotoxic constituents from the bark of Aglaia eximia (Meliaceae).
Phytochemistry Letters. 8, 28–31.
Liby, K,T., Yore, M.M., Sporn, M,B. 2007. Triterpenoids and rexinoids as
multifunctional agents for the prevention and treatment of cancer. Natural Review Cancer, 7:357–369.
Laszczyk MN. 2009. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools
in cancer therapy. Planta Medica, 75:1549– 1560.
Mullauer, F,B., Kessler, J.H., Madema, J,P., 2010. Betulinic acid, a natural compound with potent anticancer effects. Anti-Cancer Drugs, 21:215–227.
Neto, C,C. 2007. Cranberry and its phytochemicals: a review in in vitro
anticancer studies. Journal
Nutrition 137:1865–1935.
Nugroho, B. W., Edrada, R. A., Wray, V., Witte, L., Bringmann, G., Gehling, M., & Proksch, P. 1999. An insectisidal rocaglamide derivates and related compound from Aglaia odorata (Meliaceae). Phytochemistry. 51,
367-376.
Ovensná, Z., Vachalková, A., Horváthová, K., Táthová, D. 2004. Pentacyclic triterpenoic acids: new chemoprotective
compounds. Neoplasma, 51:327–333.
Pannell, C. M., 1992. Taxonnomic Monograph of the Genus Aglaia Lour. (Meliaceae). Kew Bulletin Additional Series XVI; HMSO: Kew, Richmond, Surrey, UK.
Petronelli, A., Pannitteri, G., Testa, U. 2009. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs. 20: 880–892.
Phongmaykin, J., Kumamoto, T., Ishikawa, T., Suttisri, R., Saifah, E., 2008. A new
sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch. Pharm. Res. 31, 21-27.
Phillips, D,R., Rasbery, J,M., Bartel, B., Masuda, S,P. 2006, Biosynthetic diversity in plant triterpene cyclization. Current Opin Plant Biology, 9:305–314.
Rabi, T., Bishayee, A. 2009. Terpenoids and breast cancer prevention. Breast Cancer Research Treatment, 115:223–239.
Setzer, W,N., Setzer, M,C., 2003. Plant-derived triterpenoids as potential antineoplastic agents. Mini Review Medicinal Chemistry, 3, 540–556.
Sianturi, J., Prnamasari, M., Darwati, Harneti, D., Mayanti, T.,Supratman, U., Awang, K., & Hayashi, H. 2015. New bisamide compounds from the bark of Aglaia eximia (Meliaceae). Phytochemistry Letters, 13, 297-301.
Su, B., Chai, H., Mi, Q., Riswan, S., Kardono, L.B.S., Afriastini, J. J., Santarsiero, B. D., Mesecar, A. D., Fransworth, N. R., Cordell, G. A., Swanson, S. M., & Kinghorn, D., 2006. Activity-guided isolation of cytotoxic constituents from the bark of Aglaia crassinervia collected in Indonesia. Journal of Bioorganic Medical Chemistry, 14, 960972.
91
Discussion and feedback