The The Colon Interposition as Esophageal Replacement in Long-Gap Esophageal Atresia
on
DIRECTORY OF
OPEN ACCESS
JOURNALS

Colon Interposition as Esophageal Replacement in Long-Gap Esophageal
Atresia: A Case Report
Kadek Deddy Ariyanta1*, I Made Darmajaya1, Alexandra2, Kelvin Setiawan3
-
1 Pediatric Surgery Division, General Surgery Department, Faculty of Medicine Udayana University, Sanglah General Hospital, Denpasar, Bali, Indonesia.
-
2 Pediatric Surgery Division, Departement of Pediatric Surgery, Harapan Kita Woman and Children Hospital, Jakarta, Indonesia.
-
3 General Surgery Department, Faculty of Medicine Udayana University, Sanglah General Hospital, Denpasar, Bali, Indonesia.
*Corresponding author: deddyariyanta@yahoo.com.
ABSTRACT
Background: Long-gap Esophageal Atresia (LGEA) remains one of the most challenging congenital conditions. When primary anastomoses attempts had failed, esophageal replacement (ER) is indicated in these patients. Some infants with LGEA are born with other congenital anomalies, such as rectourethral fistula. In this study, we reported our experience in managing newborn with LGEA and rectourethral fistula. Case: A 1-day-old male neonate complained of unable to swallow any breast milk and presence of feces-like discharge from external urethral orifice within 24 hours after birth. Oral gastric tube was unable to pass into the stomach and x-ray examination revealed curled gastric tube in esophagus, and there wasn’t any bubble seen from patient’s stomach. Patient then was diagnosed with long gap esophageal atresia without fistula. Esophageal replacements using left colon interposition technique was performed as closing and final procedure. Gastrostomy tube insertion, sigmoid colostomy, and cervical esophagostomy were immediately performed. Posterior sagittal anorectoplasty (PSARP) for patient’s recto-urethral fistula were performed six months after sigmoid colostomy. Patient was hospitalized with total of 32 days and gastric feeding tube can be removed three months after surgery. Conclusion: colon interposition can be safely used in long gap esophageal atreasia although patient had undergone previous colostomy repair. Long-term follow up will be needed. Further large-scale studies regarding this matter are necessary and hopefully comprehensive treatment can be established in the future.
Keywords: long gap esophageal atresia, esophageal replacements, colon interposition.
DOI: https://doi.org/10.24843/JBN.2022.v06.i02.p04
INTRODUCTION
Esophageal atresia (EA) is one of the most frequent congenital anomaly of alimentary tract, affecting 1 in 4000 birth incidences.1 EA is presented in many forms, which alter the course of the overall treatment, and it still becomes one of the most challenging and complex congenital disease for most paediatric surgeons. Between these EA forms, one form of EA stands out as the most unique and requires different approach than
55 | JBN (Jurnal Bedah Nasional)
others, which is Long-gap Esophageal Atresia (LGEA), some studies call it ‘pure atresia’.2 Although its definition still remains controversial, most paediatric surgeons agreed LGEA can be assessed in EA patients, which are not possible for primary anastomosis or failed in attempts to do so.3 Therefore, LGEA becomes one of major indications for esophageal replacements. There are several conduits previously used as esophagus substitutes, depends on the
location, the ‘gap’ between proximal and distal end of esophagus, patient’s age and other previous surgery of alimentary tract. One of the first and most commonly used techniques for esophagus replacement is colon.4
Additionally, some infants born with EA usually ‘accompanied’ with other associated anomalies. Some EA patients were born with Down syndrome, other atresia such as duodenal atresia, and other congenital abnormalities. One of those congenital anomalies is anorectal malformation, which occurred in 7% newborns with EA.5 These associated anomalies usually affect the course of the primary treatment. With so many variants regarding overall treatment of LGEA, we would like to report our experience in treating patient presenting with LGEA and rectourethral fistula. The main objective of this study is to report our experience and set of treatments in newborn baby with LGEA and anorectal malformation, in this case, rectourethral fistula. Esophageal replacements using left colon interposition technique was performed as definitive procedure.
CASE REPORT
Patient’s History and Clinical Presentation
A 1-day-old male neonate was referred to our hospital emergency department with hypersalivation and imperforate anus. Patient could not swallow any breast milk. Suctioning of oral and nose orifice were done immediately to avoid any choking from saliva ingestion to respiratory tract. Oral gastric tube was inserted to check for possible stenosis of esophagus, and tube was unable to pass into the stomach. Patient was also reported for the presence of feces-like discharge, coming from external urethral orifice within 24 hours after birth.
The patient had maternal history of polyhydramnion. Patient was born through vaginal delivery, 20 hours before admitted into our hospital. He’s the second child, born from 27-year old mother, 39 weeks of gestation with no history of other systemic and gynaecological diseases. His mother routinely went for antenatal care each month, with complete history of vaccination, and no other congenital defects were diagnosed before birth. Patient had birth weight of 2900 grams with 52 cm body length and 33 cm head circumference. His heart rate and respiratory rate were normal when admitted with no other significant findings.
Initial Treatment and Associated Congenital Anomalies
From x-ray examination, we found that the gastric tube was curled in esophagus before reaching stomach, and there wasn’t any bubble seen from patient’s stomach. Patient then was diagnosed with EA without fistula, and considered as long gap esophageal atresia. There were various agreements regarding definition and gap length in diagnosing patients with LGEA. Some author preferred using vertebral bodies in measuring the gap and some favoured using length scale (usually in cm).6 After we recognized the gap between two ends, we felt this gap would be too difficult for primary anastomoses. Therefore, we set our treatment options on esophagus replacement surgery. However, there are some other medical problems need to be taken care of first, particularly regarding patient’s daily intake and excretion route.
Soon after patient was assessed, we start our sets of treatment by creating route for patient’s intake and excretion. Gastrostomy tube insertion, sigmoid colostomy and cervical esophagostomy were immediately performed. The main objective of cervical
esophagostomy was to avoid pooling of secretions, which could be easily aspirated into respiratory tract, causing pneumonia and respiratory distress.
The second-stage procedure for patient’s recto-urethral fistula were performed six months after sigmoid colostomy. Posterior sagittal anorectoplasty (PSARP) was performed with main aim to divide rectourinary fistula followed by identification and pull-through terminal rectal pouch into normal anal position.7 Sigmoid colostomy was maintained for stool passage until the colostomy reversal, was done six months later. Two weeks after reconstructions, anal dilatations were performed routinely until appropriate anal canal diameter was achieved.
receiving meal via gastric tube. (Figure 1). Before esophageal replacement procedure was scheduled, patient underwent single contrast barium enema (SCBE) to identify any anatomical issues, particularly after patient underwent PSARP and colostomy reversal were performed and measuring colon length which will be taken as graft (Figure 2). Patient also underwent esophagogram to assessed patient’s proximal esophagus.
Patient was admitted 72 hours before surgery for routine laboratory and radiologic examination. The patient was evaluated by a team, consisting of paediatric surgeon, paediatricians, and paediatric anesthesiologists. Colonic washouts were performed every 12 hours for 3 days and repeated in the morning before surgery.
Normal saline water was washed into gastric
Esophageal Replacement Preoperative
tube every 2 hours a day prior to surgery.
Esophageal replacement procedure was
Patient was given oral antibiotic then scheduled. Patient’s weight was
(Erithromycin) 3 days prior to surgery via
measured around 14 kilograms. At that time,
gastric tube and intravenous metronidazole a patient was 2 years and 8 months old, still
day before surgery.
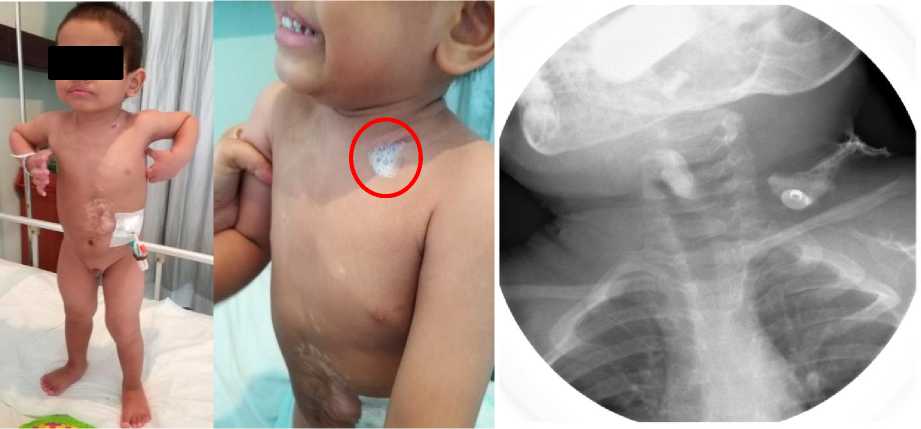
Figure 1. Patient’s condition prior to surgery, aged 2 years and 8 months old, weighted 14 kilograms. Patient had undergone cervical esophagostomy (red circle), confirmed with contrast esophagography, and still received liquid diet through gastric tube.
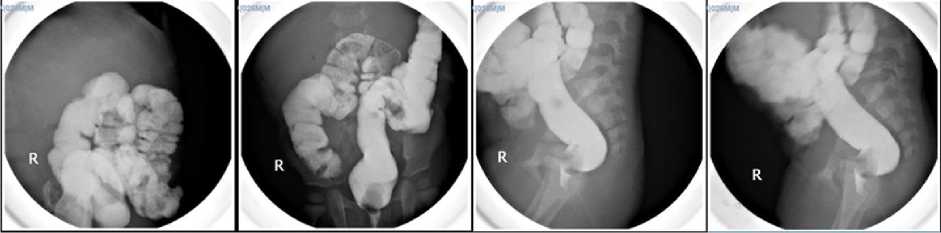
Figure 2. Single Contrast Barium Enema (SCBE) was done to ensure there were not any anatomical abnormalities, particularly after PSARP and colostomy reversal procedure.
Intraoperative
Patient was put into supine position with pillow under his shoulders to extend the neck. Gastric tube was clamped to avoid any spillage into sterile area. We started with opening abdomen through a midline incision, cutting all way through rectus abdominis muscle and peritoneum. Some adhesions were encountered when we tried to expose and mobilize the transverse colon, and adhesiolysis had to be done. After adhesions were cleared, mobilization of colon was done with precaution not to make any injury to the blood vessels. Colon mobilization was done to assess graft’s vascular supply, which is very crucial in determining graft viability and length. After detail inspection, we use our fingers to explore anterior diaphragm and approximate retrosternal tunnel to pass the graft from below. We ensured the tunnel to be wide enough to accommodate colon graft and didn’t go the wrong way. Next, we switched our attention into patient’s neck. Multiple stay sutures were applied around the proximal end of cervical esophagostomy, providing better handling (Figure 3A). Circle incision around cervical esophagostomy was made along with transverse cervical incision extended into the midline, 1 cm above manubrium sterni (Figure 3B).
Sternomastoid muscle was divided to exposed operative field in the neck. From the cervical incision, retrosternal tunnel was made and ensure the passage for colon graft.
Using long clamp from below, the instrument went through retrosternal space from abdominal incision area into anterior diaphragm, anterior pericardium, anterior thymus and exit through cervical incision opening. Later this path would be used as a passage for colon graft.
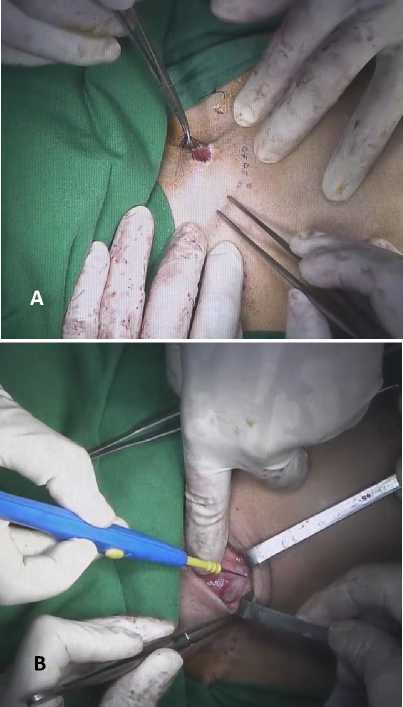
Figure 3. Multiple stay sutures (A) were applied around cervical esophagostomy proximal end for better handling and (B) were applied around cervical esophagostomy proximal end for better handling.
We decided to dissect from mid transverse colon to sigmoid, which will be used as conduit. Clamps were placed to evaluate the graft’s viability and ensured marginal vessels were sufficient enough to supply adequate circulation. After we verified graft’s length and vascularity, we ligated all the previously clamped vessels and carefully dissected the colon. Silk suture was sewn to the colon’s proximal end and pulled up into the cervical incision opening through retrosternal tunnel (Figure 4).
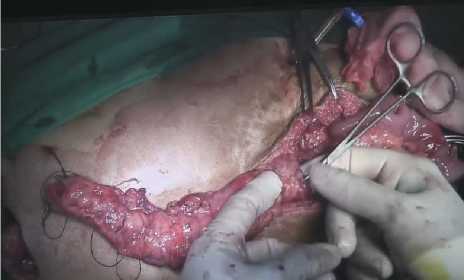
Figure 4. After colon was dissected, silk suture was sewn to the colon’s proximal end and pulled up toward cervical incision opening.
We made sure that there were no graft twists or kinks, which may disrupt graft’s blood supply. Next, primary end to end colon anastomosis was performed with linear stapler (Figure 5A). Colo-gastric anastomosis was done afterwards. Small circular incision was made close to stomach lesser curvature and dissected transverse colon was anastomosed to stomach with nonabsorbable suture (Figure 5B). Finally, esophago-colic anastomosis was performed. (Figure 5C) Both ends were cut before anastomosis to promote appropriate wound healing and graft longevity. Nasogastric tube was removed and end to end sigmoidesophagus anastomosis was successfully achieved. We evaluated both incision area and ensured there were not any leakages, foreign bodies or on-going bleeding. Warm
normal saline solution was applied to wash abdomen cavity and then both incision area was closed primarily. The operation took 4.5 hours.
Postoperative Care
Patient was admitted into paediatric intensive care unit immediately after operation. Patient stayed in the intensive care unit for 11 days and continued in the intermediate care for 3 days. Patient was still fed from gastrostomy tube for 7 days, and started clear water diet 8th day after operation. Fourteenth day after operation, patient was transferred into paediatric ward and was given milk diet via oral route. Seven days after milk diet administration, we discovered purulent discharge, leaking from his cervical wound, possibly due to esophageal fistula. This yellowish discharge was encountered from his cervical wound for 3 days. Conservative treatment was initiated immediately and patient wasn’t allowed to ingest any diet orally. After four days of fasting period, discharge was successfully reduced and clear water diet via oral route was started with strict observation. Wound care in the incision area were applied with dressings and were changed daily. After thirty-two days of hospital care, patient was discharged home.
Patient was scheduled for paediatric surgery clinic visit each week for a month, followed by monthly clinic visit. On the fourth month after operation, patient underwent esophagogram to evaluate patency of the colon graft. Mild strictures were seen at second and eight thoracic vertebrae (Figure 6) but neither anastomosis leakage nor fistula was found from the image. A month later, patients started to ingest solid food gradually. Dysphagia also occurred, but later patient was able to eat without significant difficulties.
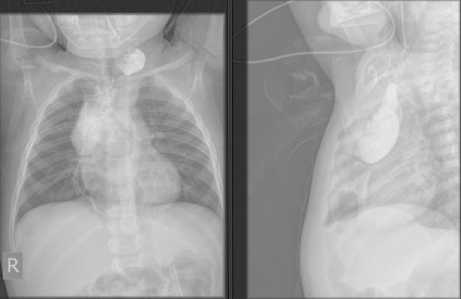
Figure 6. Patient’s esophagogram four months after colon interposition with strictures on second and eight thoracic vertebrae with deceleration of contrast towards stomach.
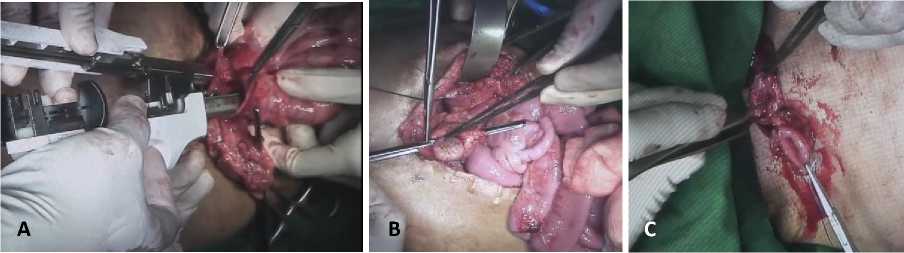
Figure 5. Colon anastomosis. (A) End to end colon anastomoses with linear stapler. (B) Cologastric anastomoses procedure. (C) Esophago-colic anastomosis through cervical incision.
DISCUSSION
Esophageal atresia (EA) remains one of the most frequent congenital anomalies found in newborn, with various incidence rates all around the world. There are various types of EA according to their anatomical descriptions. EA without tracheoesophageal fistula (TFE), particularly LGEA continues to be a major challenge, especially for paediatric surgeon. Until recently, evidences supporting comprehensive management of LGEA remain low in quantity and quality.6
Literatures regarding comprehensive treatment and guideline for those with associated congenital anomalies are also rarely published. Several case reports and studies regarding LGEA in Indonesia are already available, but based on our knowledge, this study is the first LGEA case
ever reported from Bali. In fact, this is the first LGEA case operated with colon interposition procedure in Bali.
Failure in overall organogenesis resulted in other associated anomalies in some patients with EA. Some reports suggested there are 50 % newborns with EA have at least one unrelated malformations.5 In 2016, a descriptive study by Bairdain stated the most common isolated anomalies found in patient with LGEA were cardiac (44%) renal, and vertebral malformations (25% each). The study also mentioned that VACTERL (vertebral, anal, cardiac, tracheoespohageal, renal and limb defects) were occurred more frequent in patients with non-LGEA compared to LGEA patients. Coincidentally, anorectal malformations were also more common in non-LGEA group.8
LGEA is one of the major indications for esophageal replacement. Patient’s own esophagus remains the best conduit but basically, if primary anastomoses could not be achieved, or failed to do so, other conduit for esophageal replacement are needed. There are numerous types of esophageal replacement procedures, including colon interposition, gastric tube esophageal replacement, jejunal interposition and gastric interposition. Each had their own indications and advantages, but colon interposition remains as a standard technique and the most commonly used technique in bridging the
gap.4 However, based on Ionescu et al statements, gastric tube esophageal replacement is preffered option in patients with history of previous colostomy.3 Again, the choice of replacement technique depends upon each surgeon’s experience and preference, while we have never performed any esophageal replacement procedure in our hospital before. Options regarding usage of either right colon or left colon are also based on surgeon’s experience and intraoperative evaluation, particularly the gross visual of colon vascularity and possibility for colon dissection.5
Based on complication rate, Daniel von Allmen stated in his study in 2015 that colon interposition had 55-61% of complications rate, second most often after gastric interposition (22-78%) Anastomoses leakage became the most problematic issues found shortly after surgery. (22-36%) Primary disadvantages of this procedure is mainly because this procedure needs at least three anastomoses.9 These three anastomoses are usually hand sown, however some health centers are using gastrointestinal anastomosis stapler, basically to help shortening operation duration.10 Strictures at proximal anastomosis are also common complications found in colon interposition (11-49%).9 These strictures were managed with endoscopic dilatation, however some patients require surgical revision if dilatation attempts had failed.3 A study by Laohapensang in 2019 revealed 3 patients who underwent colonic interposition and all three had anastomotic stenosis (100%) but all resolved after endoscopic dilatation.11 Same stenosis problem was seen on our patient, but patient will be monitored in every follow-up examinations. Further action, such as endoscopic dilatation, will be considered, if the problem persists.
In our report, patient was hospitalized for 32 days, 14 days in intensive and intermediate care units and 18 days in paediatric ward. According to Laohapensang’s report, three patients who underwent colonic interposition had admission period ranged between 20 and 55 days, with intensive care period between 4 and 14 days.11 In case of long term complications, dysphagia symptoms were noted in 4 studies from Stefano’s systematic review in 2017.12 According to a long-term quality of life study conducted by Christina et al, dysphagia rarely occurred, counted for only 11% from 63 patients.13 Unfortunately, dysphagia was reported from our patient, but due to his age, he was still unable to describe it clearly. We are hoping this symptom will reside eventually without affecting his quality of life in the future.
CONCLUSION
Long gap esophageal atresia (LGEA) still remains as one of the most challenging birth anomalies found in newborns, Paediatric surgeons are required to repair primarily, since patient’s own esophagus is the best conduit. However, when the gap’s distance makes it difficult for primary repair or previous attempts had failed, esophageal replacement methods are indicated. There are many methods, which are already introduced and can be used as esophageal substitutes, such as colon interposition, gastric tube replacement, jejunal interposition and gastric interposition. Each of these methods had their own pros and cons, and we prefer using left colon interposition method as replacement. In this report, we had presented a case of LGEA newborn with congenital anorectal malformations. There were limited studies regarding management of LGEA with anorectal anomalies, particularly the safety of
colon interposition usage in patients with history of sigmoid colostomy.
Based on patient’s condition post-operatively, we can conclude that colon interposition can be safely used, although patient had undergone previous colostomy repair. Patient was hospitalized with total of 32 days, and gastric feeding tube can be removed three months after surgery. Patient encountered esophageal fistula 5 days after operation, and was successfully treated conservatively. Patient can safely took liquid food from mouth 10 days after surgery and start eating solid food 5 months after surgery. Strictures, as the most common complications post-operatively, were encountered in several spots and somehow interfered with patient’s swallowing process. Long-term follow up will be needed and updates will be reported soon. Further large-scale studies regarding this matter are necessary and hopefully comprehensive treatment can be established in the future.
ACKNOWLEDGEMENT
The author would like to thank family, friends and supervisor for guidance, critics and suggestions to this manuscript.
DISCLOSURE
Authors declare no conflict of interest of this study.
REFERENCES
-
1. Nassar N, Leoncini E, Amar E, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2015;94:893-9.
-
2. Liu J, Yang Y, Zheng C, et al. Surgical outcomes of different approaches to esophageal replacement in long-gap esophageal atresia: A systematic review. Medicine (Baltimore). 2017;96:e6942.
-
3. Ayad MA-L, El-Asmer, Khaled Mohamed AFH. Colon (Including Ileum) Interposition. In: Till H, Thomson M, Foker JE, Holcomb GW, et al, editors. Esophageal And Gastric Disorders in Infancy and Childhood. 1st ed. Berlin: Springer International Publishing; 2017. p. 347-59.
-
4. Snyder C, Colombani PM, Chandler N. The Esophagus. In: Holcomb III GW, Murphy JP, Peter SDS, et al, editors. Holcomb and Ashcraft’s Pediatric Surgery. 7th ed. Edinburg: Elsevier Inc.; 2020. p. 430-4.
-
5. Spitz L, Coran AG. Esophageal Replacement. In: Coran AG, Adzick NS, Krummel TM, et al, editors. Pediatric Surgery. 7th ed. Philadelphia: Elsevier Inc.; 2012. p. 927-32.
-
6. Committee P, Baird R, Lal DR, et al. Management of long gap esophageal atresia: A systematic review and
evidence-based guidelines from the APSA Outcomes and Evidence Based. J Pediatr Surg. 2019;54:675-87.
-
7. Chung DH. Pediatric Surgery. In: Townsend CM, Beauchamp RD, Evers BM, et al, editors. Sabiston Textbook of Surgery. 20th ed. Philadelphia: Elsevier Inc; 2017. p. 1879-80.
-
8. Bairdain S, Zurakowski D, Vargas SO, et al. Long-Gap Esophageal Atresia Is a Unique Entity within the Esophageal Atresia Defect Spectrum. Neonatology. 2017;111:140-4.
-
9. Allmen D Von, Wijnen RMH. Bridging the Gap in the Repair of Long-Gap Esophageal Atresia: Still Questions on Diagnostics and Treatment. Eur J Pediatr Surg. 2015;25:312-7.
-
10. Eze JC, Ezemba N, Onyekwulu FA, et al. Right colon interposition in corrosive esophageal long segment stricture: Our local experience. Niger J Clin Pract.
2014;17:314-9.
-
11. Laohapensang M, Tangsriwong T, Tantemsapya N. Esophageal Replacement in Children: A 10-Year , Single-Center Experience. Siriraj Med J. 2019;71:80-8.
-
12. Garritano S, Irino T, Scandavini CM, Tsekrekos A, Lundell L, Rouvelas I. Long-term functional outcomes after
replacement of the esophagus in pediatric patients: A systematic literature review. J Pediatr Surg. 2017;52:1398-408.
-
13. Greene CL, DeMeester SR, Augustin F, et al. Long-term quality of life and alimentary satisfaction after
esophagectomy with colon interposition. Ann Thorac Surg. 2014;98:1713-20.
63

Discussion and feedback