PHYTOCHEMICAL ANALYSIS OF Mucuna pruriens L. LEAVES EXTRACT AND ITS INHIBITION TEST AGAINST Curvularia lunata (WAKK). BOED. THAT CAUSED LETTUCE LEAF SPOT
on
Phytochemical Analysis Of Mucuna Pruriens L. Leaves Extract And Its Inhibition Test Against Curvularia Lunata (Wakk). Boed.
That Caused Lettuce Leaf Spot
Nata, IMAP., Suada, IK., Kharitonov, S. & Phabiola, TA.
PHYTOCHEMICAL ANALYSIS OF Mucuna pruriens L. LEAVES
EXTRACT AND ITS INHIBITION TEST AGAINST Curvularia lunata
(WAKK). BOED. THAT CAUSED LETTUCE LEAF SPOT
I Made Ary Putra Nata1, I Ketut Suada1*, Sergey Kharitonov2, Trisna Agung Phabiola1 1Department of Agroecotechnology, Faculty of Agriculture, Udayana University 2Department of Soil Geography, Faculty of Soil Science, Lomonosov Moscow State University *Email: ketutsuada@unud.ac.id
ABSTRACT
Curvularia lunata is a pathogenic fungi that attack various plant species including lettuce. Its control needs to be carried out in an environmentally friendly manner, namely using botanical fungicides. M. pruriens is a Received: legume plant that is widespread in Indonesia that has a potential as a
botanical fungicide, beside as a traditional medicine. The purpose of this 23 September 2022 study was to study the chemical content of M. pruriens leaves and its potency to inhibit the growth of C. lunata. The determination of the compounds was carried out by Gas Chromatography-Mass Spectrophotometry and the inhibition test was carried out using diffusion well, and agar dillution method. The result showed that the crude extract of M. pruriens leaves contained ethyl isothiocyanate that act as an antifungal with AUC of 20,78%. The MIC of the extract was 0,9%. The extract at 5% concentration was capable to inhibit C. lunata growth, mycelium mass, and conidia production respectively 38,33%; 67,14%; 99,88% compared to control.
Accepted:
5 October 2022
Published:
8 October 2022
Keywords: Mucuna pruries, phytochemical, Curvularia lunata, antifungal
INTRODUCTION
Curvularia lunata is a pathogenic fungus that attacks various types of plants (Garcia-Aroca et al., 2018), including lettuce. The highlands climatic conditions that have high humidity are optimal conditions for
pathogenic fungi to grow. C. lunata is one of the important pathogens that cause leaf spots in lettuce especially in nurseries and causes extensive losses (Baiyee et al., 2018). The first report on lettuce was in Thailand in 2018 (Pornsuriya et al., 2018). C. lunata spreads
through the soil and seeds (Akinbode, 2010; Watanabe, 2010). The application of synthetic fungicides is the most effective control technique for controlling C. lunata. Mancozeb-based fungicides are the most effective fungicides (Abrar et al., 2020).
Long-term application of mancozebbased fungicides will cause C. lunata to be resistant against this fungicide (Susanto and Prasetyo, 2013). Therefore, environmentally friendly control is needed, namely with botanical fungicides. Mucuna pruriens is a legume plant that is widespread in Indonesia (Mulyani et al., 2016). M. pruriens is often used as a traditional medicine traditional (Sathiyanarayanan and Arulmozhi, 2007), in addition, M. pruriens also has the potential to be a botanical fungicide because it has antifungal properties (Nidiry et al., 2011). Nidiry et al. (2011) in their study state that M. pruriens seeds extractive was able to inhibit Colletotrichum sp. and Fusarium solani. Khan et al. (2008) also state that M. pruriens seed extract from various solvent showed antifungal activity against Aspergillus niger.
Study conducted by Rayavarapu et al. (2011) found that the whole plant of M. pruriens extract was able to inhibit the growth of Curvularia lunata, Fusarium oxysporum, Pencillium expansum, Rhizoctonia solani,
Tiarosporella phaseolina, Ustilago maydis. The aim of this study was to determine the content of compounds in the leaf extract of M. pruriens and its potential in inhibiting the growth of C. lunata.
MATERIAL AND METHOD
The equipments used in this study were autoclave, laminar air flow cabinet, vacuum rotary evaporator, gas chromatography-mass spectrophotometry, haemacytometer, microscope, blender, test tubes, petri dishes, erlemeyers, beaker glass, measuring cups, sprayers, micropipette, tweezers, scalpels, ose needles, cork borer, calipers, stationery, and camera. The materials used in this study were M. pruriens leaves and lettuce leaves that were symptomatic of C. lunata attacks. Other materials used are aquades, PDA (Potato Dextrose Agar) media, alcohol (70%) Pro Analyst Methanol MERCKTM, HCl (10%), aluminum foils, plastic wraps, Whatman No.2 filter papers, tissue papers, and labels.
Extract preparation
M. pruriens leaves samples were taken in South Denpasar. The leaves then washed and cleaned and then airdried for 10 days. The dried leaves then grinded with a blender to get simplisia. The leaf simplisia then macerated with methanol Pro Analyst MERCKTM in a
ratio of 1:5 for 5x24 hours and stirred periodically. The solution then filtered using Whatman No.2 filter paper. Macerate concentrated with a vacuum rotary evaporator to obtain a crude extract.
Compound analysis
Analysis of M. pruriens leaf extract compounds was carried out at the Bidlabfor Forensic Laboratory of the Bali Regional Police Departement. The extract was analyzed with Agilent 7890 MSD 5977B type Chromatography-Mass Spectrophotometry (GC-MS) Gas, with a Wakosil ODS/5C18-200 silica column with a size of 4.6 x 200 mm using N2 gas as a career. The injection temperature used was 290oC for 27 minutes with an injection rate of 1 ml/min.
Culture media preparation
PDA media is made by boiling 200 g of potatoes in 1 L of aquades for 1 hour. The boiled water of the potatoes then filtered and added 20 g of dextrose, 15 g of agar, and 0.5 g of chloramphenicol, then stirred until dissolved. The media was sterilized using an autoclave at a temperature of 121oC for 15 minutes. The sterile media then allowed to stand so that the temperature becomes 40oC and then poured into a sterile petri dish.
Pathogen isolation and identification
Symptomatic lettuce leaves were taken and sterilized using 70% alcohol on the part between the symptomatic and healthy part. The leaves were cut by 0.5 cm x 0.5 cm and then placed on a solid PDA medium and incubated for 5 days at a temperature of 25oC. The growing fungus then purified. Once pure, isolates were observed under a microscope and identified based on their morphology following the Barnett and Hunter (1998) identification key.
Determination of minimum inhibitory concentration
Determination of Minimum Inhibitory Concentration was carried out by diffusion wells method with triplicate. The tested concentration was 0%; 0,1%; 0,2%; 0,3%; 0,4%; 0,5%; 0,6%; 0,7%; 0,8%; 0,9%; 1%; 1,1%; 1,2%; 1,3%; 1,4%; 1.5% and aquades as control. The extract is dissolved with 80% Tween 10% solution to obtain the test concentration. A total of 0.5 ml of spore suspension was poured in 20 ml of diluted PDA and shaken until evenly distributed in a petri dish. Wells are made using a cork borer with 5 mm diameter. Each well was filled with 20 μl of extract solution. The media then stored in a refrigerator at a temperature of 4oC
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY ∙ Vol. 10 No. 1 ∙ September 2022
for 2 hours, then incubated at a temperature of 25oC for 2 days.
Inhibition test assay
The inhibition test against the growth of C. lunata was carried out by agar dilution method. The design used was a Complete Randomized Design with four replicate. The observed variables were colony growth, mycelium mass, and the number of conidia. The concentration of the extract tested was 0.1%; 0,2%; 0,5%; 1%; 2%; 5%; and 0% as a
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2022.v10.i01.p06
control. A colony of C. lunata with 5 mm in diameter was placed in the center of a solid PDA medium that already contains the extract. Observations were made as the control fungus fully grew to the petri. For colony growth assay, the diameter of the colony was measured, then the percentage of colony growth inhibition of the extract is calculated by equation (1).
control diameter - treatment diameter Inhibiton rate (%) = × 100%
control diameter
(1)
For the mass of mycelium assay, the mycelium of C. lunata was cleaned from the media by pouring heated 10% HCl, then filtered using filter paper. The mycelium then dried at a temperature of 80oC until its weight constant, then weighed. For the amount of conidia formed, conidia were harvested by pouring 20 mL of aquades, then dredged with an ose needle so that a conidia suspension
obtained. The number of conidia was calculated using a haemacytometer.
Data analysis
The data obtained were quantitatively analyzed using analysis of variance (ANOVA) at a level of 5% and then analyzed using the Least Significant Difference (LSD) test with a level of 5%.
RESULT AND DISCUSSION
Maceration of 0.5 kg of simplisia leaves of M. pruriens produced a rendement of 4.13%, which is 20.63 g of green-yellow-
black crude extract. The crude extract obtained then analyzed using GC-MS and tested its antifungal activity. The results of the
analysis of the content of compounds in the leaf extract of M. pruriens using GC-MS
showed that there were 22 compounds contained in the extract shown in Figure 1.
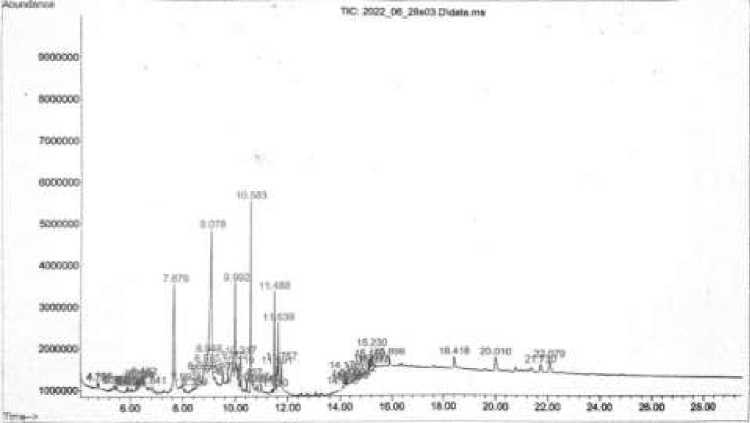
Figure 1 Chromatogram of M. pruriens leaves extract
Compounds contained in the leaf extract of M. pruriens were, 3-propoxypropan-1-amine; N-butylacetamide; 2-oxobutanoic acid; D-galactonic acid, γ-lactone; phenylephrine; β-D-glucosyloxyazoxymethane; 3-(1-isopropyl-but-3-enyloxy)-butyric acid; 9,11-octadecadiyonic acid,8-hydroxy-, methyl ester; ethyl isothiocyanate; inositol; D-galactose; heptadecanal; 1,2-15,16-
diepoxyhexadecane; hexadecanoic acid, methyl ester; N-hexadecanoic acid; tetraacetyl-d-xylonic nitrile; phytol; 9,12-octadecadienoic acid (Z,Z)-; linoelaidic acid; 9,12,15-octadecatrien-1-ol; 9,12,15-octadecatrienoic acid, (Z,Z,Z)-; and octadecanoic acid. The main compounds contained in the of M. pruriens leaves crude extract are presented in Table 1.
Table 1. Primary compunds found in M. pruriens leaves extract
|
No. |
Compund Name |
AUC (%) |
Rf |
|
1 |
N-butylacetamide |
11,31 |
7.679 |
|
2 |
2-oxobutanoic acid |
11,31 |
7.679 |
|
3 |
Ethyl isothiocyanate |
20,78 |
9.078 |
|
4 |
Inositol |
10,51 |
9.992 |
|
5 |
D-galactose |
10,51 |
9.992 |
|
6 |
N-hexadecanoic acid |
8,33 |
10.583 |
The main component contained in the M. pruriens leaves crude extract was ethyl isothiocyanate with an AUC value of 20.78% at a retention time of 9,078, this compound has an antifungal property (Smolinska et al., 2003; Kurt et al., 2011; Wu et al., 2011; Kara and Soylu, 2020). N-butylacetamide and 2-oxobutanoic acid are the second most constituent compounds found in the extract with an AUC ratio of 11.31% at a retention time of 7,679. Successively these compounds function as insect repellents (Debboun et al., 2015) and flavorings (PubChem, 2004). The third highest content were inositol and D-galactose at a time of 9,992 with an AUC value of 10.51%. Inositol often used in the treatment of Polycytic ovary syndrome
(PCOS) (Holub, 1986; Levine, 1997; Nestler et al., 1999; Sacchinelli et al., 2014; Unfer et al., 2012), whereas D-galactose used as a senescent inducer in the study of aging models in animals (Bo-Htay et al., 2018; Shwe et al., 2018). N-hexadecanoic acid was also a compound that contained in the M.pruriens leaves crude extract with an AUC value of 8.33% at a retention time of 10,583.
This compound has anti-inflammatory activity (Aparna et al., 2012). Minimum inhibitory concentration (MIC) was determined to determine the minimum concentration of M. pruriens leaves crude extract that were able to inhibit fungal growth. The results of the MIC test are presented in Table 2.
Table 2. MIC of M. pruriens leaves crude extract against C. lunata
|
No |
Extract concentration (%) |
Inhibition zone diameter (mm) |
|
1 |
0 |
0 |
|
2 |
0,1 |
0 |
|
3 |
0,2 |
0 |
|
4 |
0,3 |
0 |
|
5 |
0,4 |
0 |
|
6 |
0,5 |
0 |
|
7 |
0,6 |
0 |
|
8 |
0,7 |
0 |
|
9 |
0,8 |
0 |
|
10 |
0,9* |
0,57 |
|
11 |
1 |
1,33 |
|
12 |
1,1 |
1,63 |
|
13 |
1,2 |
1,83 |
|
14 |
1,3 |
2,27 |
|
15 |
1,4 |
2,63 |
|
16 |
1,5 |
3,03 |
|
17 |
Aquades |
0 |
Description: *MIC
The test results of M. pruriens leaves crude extract showed that the minimum concentration of the extract to be able to inhibit the growth of C. lunata was at a concentration of 0.9%. This is shown in Figure 2, namely with the formation of an inhibitory zone at a concentration of 0.9% after 2 days of incubation.
The M. pruriens leaves crude extract had a weak inhibitory power against the growth of C. lunata due to the diameter of the inhibition zone formed less than 5 mm (Davis and Stout, 1971). This could be because the concentration of antifungal compounds
contained in the crude extract of the leaves of M. pruriens was quite low. According to Suprapta (2014), plant extracts that had a minimum inhibitory concentration above 0.5% are less suitable to be used as botanical fungicides. This is because the content of active ingredients contained in the extract is quite low so that it requires a lot of ingredients to make it and is considered less practical and economical, as well as the use in high concentrations of plants has the potential to cause phytotoxic. The diffusion power of the extract also thought to be a factor in the
lack of strength of the extract in inhibiting C.
lunata (Rios et al., 1988).
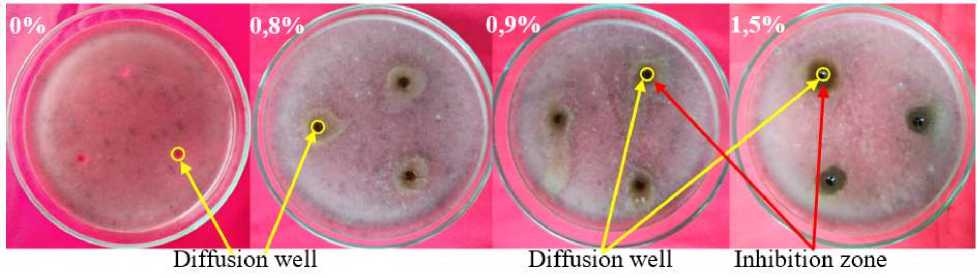
Figure 2 MIC of M. pruriens leaves crude extract against C. lunata
The ANOVA analysis of the inhibition test showed that the treatment of differences in the concentration of M. pruriens leaves crude extract had a significant effect on the average colony diameter, the percentage of
inhibitory power, the average weight of the mycelium, and the density of spores. The results of the test of inhibitory power of the M. pruriens leaves crude extract against C. lunata are presented in Table 3.
Table 3 Inhibitory power of M. pruriens leaves crude extract against C. lunata
|
No |
Extract concentration (%) |
Average diameter of colony (cm) |
Colony growth inhibition rate (%) |
Average mycelium mass (mg) |
Mycelium mass shrinkage rate (%) |
Conidia density (105 CFU/ml) |
Conidia inhibition rate (%) |
|
1 |
0% |
9,00a |
0,00a |
225,78a |
0,00a |
2,138a |
0,00a |
|
2 |
0,1% |
9,00a |
0,00a |
202,43b |
10,17b |
1,382b |
25,54b |
|
3 |
0,2% |
8,95a |
0,56a |
184,20bc |
18,40c |
1,075b |
44,41b |
|
4 |
0,5% |
8,75a |
2,78b |
164,03c |
27,45d |
0,557c |
70,03c |
|
5 |
1% |
8,58a |
4,72b |
131,00d |
42,15e |
0,223cd |
88,18cd |
|
6 |
2% |
6,40b |
28,89c |
93,95e |
58,38f |
0,090cd |
94,98d |
|
7 |
5% |
5,55c |
38,33d |
74,30e |
67,14g |
0,003d |
99,88d |
Information: Numbers followed by the same letter in the same column are not significant based on the LSD test at the level of 5%
Based on Table 2, the extract concentration of 0.1% still had not inhibited the growth of C. lunata colonies. Growth inhibition of C. lunata colonies occured at an extract concentration of 0.2%; 0,5%; 1%; 2%; and 5% with a successive inhibitory power
value of 0.56%; 2,78%; 4,72%; 28,89%; and 38.33%. The results of the test of the inhibitory power of M. pruriens leaves crude extract against the growth of C. lunata colonies are shown in Figure 3.

Figure. 3 Inhibitory power of M. pruriens leaves crude extract against C. lunata colonies growth
In addition to inhibiting the growth of C. lunata colonies, the extract was able to reduce the weight of the mycelium and inhibit the formation of C. lunata conidia. Based on Table 2, the extract was able to reduce the weight of C. lunata mycelium ranging from 0.1% to 5% concentrations with values ranging from 10.17% to 67.14% and inhibiting the formation of C. lunata conidia
ranging from 0.1% to 5% concentrations with values ranging from 25.54% to 99.88%.
The inhibition test results showed that the M. pruriens leaves crude extract was able to inhibit the growth of C. lunata which was shown by decreasing the diameter of the colony, the weight of the mycelium, and also the conidia produced by the fungus. Crude extract of M. pruriens leaves was able to
inhibit the growth of C. lunata allegedly because the extract contains ethyl isothiocyanate. Based on the results of the GC-MS that had been carried out (Table 1), the crude leaf extract of M. pruriens contains ethyl isothiocyanate. Ethyl isothiocyanate is a compound that has antifungal activity (Kara and Soylu, 2020; Kurt et al., 2011; Smolinska et al., 2003; Wu et al., 2011)
The most effective extract concentration in inhibiting the growth of C. lunata was the extract concentration of 5% with the inhibitory power of the colony reaching 38.33%. This is supported by research conducted by Kara and Soylu, (2020), Kurt et al. (2011), and Yang et al. (2021). In addition, at the same concentration, the mycelium of C. lunata shrank by 67.14%. This is thought to be due to morphological alterations in C. lunata. Compounds of the isothiocyanate group are able to damage the morphology of fungi (Kara & Soylu, 2020; Yang et al., 2021). Crude extract of M. pruriens leaves with a concentration of 5% also caused C. lunata to produced almost no conidia. This suggests that the crude extract of the leaves of M. pruriens not only inhibits the growth of fungi, but was also able to inhibit the formation of conidia. This also reported by Otoni et al. (2014) and Yang et al.
(2021). According to Sudana (1997), the formation of conidia in pathogenic fungi is one of the important factors in the spread and infectious rate of plant diseases. Therefore, compounds capable of inhibiting the formation of conidia are very well used as the active ingredients of fungicides.
Crude extract of M. pruriens leaves was able to inhibit the growth of C. lunata, this in line with Rayavarapu et al. (2011). Thanks to the ethyl isothiocyanate that contained in the extract. Ethyl isothiocyanate is a compound that belongs to the group of isothiocyanate. Isothiocyanate is a volatile compound that is one of the derivatives of glucosinolate which is generally contained in plants of the family Brassicae (Plaszkó et al., 2021). Compounds from the isothiocyanate group have been widely studied for their antifungal activity (Kara & Soylu, 2020; Kurt et al., 2011; Otoni et al., 2014; Park et al., 2013; Smolinska et al., 2003; Wu et al., 2011; Yang et al., 2021).
Gluthatione, one of natural antioxidant that produced by living organism (Aguirre et al., 2006; Pócsi et al., 2004), often become the target of isothiocyanate group compounds. (Plaszkó et al., 2021). This also stated by Schreiner and Koide (1993). It can be said that isothiocyanate compounds can cause oxidative stress in fungi because the amount
of gluthatione is reduced resulting in accumulation Reactive Oxygen Species ROS, this is in line with research conducted by Bertóti et al. (2016). Oxidative stress can lead
to the occurrence of protein dysfunction, protein oxidation, enzyme inhibition, lipid peroxidation, as well as DNA damage (Aguirre et al., 2006; Pócsi et al., 2004).
CONCLUSION
Crude extract of M. pruriens leaves contains 22 compounds with the most compounds, namely ethyl isothiocyanate which is antifungal. The inhibitory power of the M. pruriens leaves crude extract against
REFERENCE
Abrar, M., Hassan, U., Butt, I., Khan, I. H., Javaid, A., & Shad, N. (2020).
Comparative efficacy of three fungicides for in vitro control of Curvularia lunata. Research Article Mycopath, 18(2), 47–52.
Aguirre, J., Hansberg, W., & Navarro, R. (2006). Fungal responses to reactive oxygen species. Medical Mycology, 44(1), 101–107.
https://doi.org/10.1080/1369378060090 0080
Akinbode, O. A. (2010). Evaluation of antifungal efficacy of some plant extracts on Curvularia lunata, the causal organism of maize leaf spot. African Journal of Environmental Science and Technology, 4(11), 797–800.
http://www.academicjournals.org/AJES T
Aparna, V., Dileep, K. v., Mandal, P. K., Karthe, P., Sadasivan, C., & Haridas, M. (2012). Anti-inflammatory property of n-hexadecanoic acid: structural
C. lunata was weak with a MIC value of 0.9% and was able to inhibit colony growth, reduce the weight of the mycelium, and inhibit the formation of C. lunata conidia by 38,33%; 67,14%; and 99,88% respectively compared to control.
evidence and kinetic assessment.
Chemical Biology and Drug Design, 80(3), 434–439.
https://doi.org/10.1111/j.1747-0285.2012.01418.x
Baiyee, B., Pornsuriya, C., Ito, S. ichi, & Sunpapao, A. (2018). Trichoderma spirale T76-1 displays biocontrol activity against leaf spot on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biological Control, 129, 195–200. https://doi.org/10.1016/j.biocontrol.201 8.10.018
Barnett, H. L., & Hunter, B. B. (1998). Illustrated Genera of Imperfect Fungi (4th ed.).
Bertóti, R., Vasas, G., Gonda, S., Nguyen, N. M., Szőke, É., Jakab, Á., Pócsi, I., & Emri, T. (2016). Glutathione protects Candida albicans against horseradish volatile oil. Journal of Basic Microbiology, 56(10), 1071–1079.
https://doi.org/10.1002/jobm.20160008 2
Bo-Htay, C., Palee, S., Apaijai, N., Chattipakorn, S. C., & Chattipakorn, N.
(2018). Effects of d-galactose-induced ageing on the heart and its potential interventions. Journal of Cellular and Molecular Medicine, 22(3), 1392–1410. https://doi.org/10.1111/jcmm.13472
Davis, W. W., & Stout, T. R. (1971). Disc plate method of microbiological antibiotic assay. Applied Microbiology, 22(4), 659–665.
Debboun, M., Frances, S. P., & Strickman, D. A. (2015). Insect Repellents Handbook (2nd ed.).
Garcia-Aroca, T., Doyle, V., Singh, R., Price, T., & Collins, K. (2018). First report of Curvularia leaf spot of corn, caused by Curvularia lunata, in the United States. Plant Health Progress, 19(2), 140–142. https://doi.org/10.1094/PHP-02-18-0008-BR
Holub, B. J. (1986). Metabolism and function of myo-inositol and inositol phospholipids. Ann. Rev. Nutr, 6, 563– 597.
Kara, M., & Soylu, E. M. (2020). Assessment of glucosinolate-derived isothiocyanates as potential natural antifungal compounds against citrus sour rot disease agent Geotrichum citri-aurantii. Journal of Phytopathology, 168(5),
279–289.
https://doi.org/10.1111/jph.12889
Khan, A. K., Koneru, A., Kumar, P. K., S., S., Kumar, E., & K., S. (2008). Antifungal and antihelmintic activity of extracts of Mucuna pruriens seeds. Phamacology Online, 2(79), 776–780.
Kurt, Ş., Güneş, U., & Soylu, E. M. (2011). In vitro and in vivo antifungal activity of synthetic pure isothiocyanates against Sclerotinia sclerotiorum. Pest
Management Science, 67(7), 869–875. https://doi.org/10.1002/ps.2126
Levine, J. (1997). Controlled trials of inositol in psychiatry. European
Neuropsychopharmacology, 7, 147–
155.
Mulyani, L., Kartadarma, E., & Fitrianingsih, S. P. (2016). Manfaat dan kandungan kacang kara benguk (Mucuna pruriens L.) sebagai obat. Prosiding Farmasi, 351–357.
Nestler, J. E., Jakubowicz, D. J., Reamer, P., Gunn, R. D., & Allan, G. (1999).
Ovulatory and metabolic effects of d-chiro-inositol in the polycystic ovary syndrome. The New England Journal of Medicine, 340(17), 1314–1320.
Nidiry, E. S. J., Ganeshan, G., & Lokesha, A.
N. (2011). Antifungal activity of Mucuna pruriens seed extractives and L-dopa. Journal of Herbs, Spices and Medicinal Plants, 17(2), 139–143.
https://doi.org/10.1080/10496475.2011. 581135
Otoni, C. G., Soares, N. D. F. F., da Silva, W. A., Medeiros, E. A. A., & Baffa Junior, J. C. (2014). Use of allyl isothiocyanate-containing sachets to reduce Aspergillus flavus sporulation in peanuts. Packaging Technology and Science, 27(7), 549– 558. https://doi.org/10.1002/pts.2063
Park, H.-W., Choi, K.-D., & Shin, I.-S.
(2013). Antimicrobial activity of isothiocyanate (ITCs) extracted from horseradish (Armoracia rusticana) root against oral microorganisms.
Biocontraol Science, 18(3), 163–168.
Plaszkó, T., Szűcs, Z., Vasas, G., & Gonda, S.
(2021). Effects of glucosinolate-derived isothiocyanates on fungi: A
comprehensive review on direct effects, mechanisms, structure-activity
relationship data and possible agricultural applications. Journal of Fungi, 7(539).
https://doi.org/10.3390/jof7070539
Pócsi, I., Prade, R. A., & Penninckx, M. J.
(2004). Glutathione, altruistic
metabolite in fungi. Advances in
Microbial Physiology, 49, 1–76.
https://doi.org/10.1016/S0065-2911(04)49001-8
Pornsuriya, C., Ito, S. I., & Sunpapao, A. (2018). First report of leaf spot on lettuce caused by Curvularia aeria. Journal of General Plant Pathology, 84(4), 296–299.
https://doi.org/10.1007/s10327-018-0782-7
PubChem. (2004). 2-Oxobutanoic acid.
https://pubchem.ncbi.nlm.nih.gov/comp ound/58
Rayavarapu, K. A., Apparaorayavarapu, K., & Kaladhar, D. (2011). Evaluation of antimicrobial activity of Mucuna pruriens on plant pathogens. Asian Journal of Biochemical and Pharmaceutical Research, 1(2): 593
600.
Rios, J. L., Recio, M. C., & Villar, A. (1988). Screening methods for natural products with antimicrobial activity: a review of literature. Journal of
Ethnopharmacology, 23, 127–149.
Sacchinelli, A., Venturella, R., Lico, D., di Cello, A., Lucia, A., Rania, E., Cirillo, R., & Zullo, F. (2014). The efficacy of inositol and n-acetyl cysteine administration (ovaric hp) in improving the ovarian function in infertile women with PCOS with or without insulin resistance. Obstetrics and Gynecology International, 2014, 1–5.
https://doi.org/10.1155/2014/141020
Sathiyanarayanan, L., & Arulmozhi, S.
(2007). Mucuna pruriens Linn.-a comprehensive review. Pharmacognosy Reviews, 1(1), 157–162.
Schreiner, R. P., & Koide, R. T. (1993). Mustards, mustard oils and mycorrhizas. New Phytol, 123, 107–113.
Shwe, T., Pratchayasakul, W., Chattipakorn, N., & Chattipakorn, S. C. (2018). Role of D-galactose-induced brain aging and
its potential used for therapeutic interventions. Experimental
Gerontology, 101, 13–36.
https://doi.org/10.1016/j.exger.2017.10. 029
Smolinska, U., Morra, M. J., & Knudsen, G. R. (2003). Isothiocyanates produced by brassicaceae species as inhibitors of Fusarium oxysporum. Plant Disease, 87(4), 407–412.
Sudana, I. M. (1997). Metabolit sekunder baru dari Pseudomonas flourescens efektif mengendalikan Ceratocytis paradoxa pada tanaman kelapa. Bandung.
Suprapta, D. N. (2014). Pestisida Nabati: Potensi dan Prospek Pengembangan. Pelawa Sari: Denpasar.
Susanto, A., & Prasetyo, A. (2013). Respons Curvularia lunata penyebab penyakit bercak daun kelapa sawit terhadap berbagai fungisida. Jurnal Fitopatologi Indonesia, 9(6), 165–172.
https://doi.org/10.14692/jfi.9.6.165
Unfer, V., Carlomagno, G., Dante, G., & Facchinetti, F. (2012). Effects of myoinositol in women with PCOS: A systematic review of randomized controlled trials. Gynecological Endocrinology, 28(7), 509–515.
https://doi.org/10.3109/09513590.2011. 650660
Watanabe, T. (2010). Pictorial atlas of soil and seed fungi: morphologies of cultured fungi and key to species. CRC Press/Taylor & Francis.
Wu, H., Zhang, X., Zhang, G. A., Zeng, S. Y., & Lin, K. C. (2011). Antifungal vapourphase activity of a combination of allyl isothiocyanate and ethyl isothiocyanate against Botrytis cinerea and Penicillium expansum infection on apples. Journal of Phytopathology, 159(6), 450–455.
https://doi.org/10.1111/j.1439-0434.2011.01792.x
Yang, B., Li, L., Geng, H., Zhang, C., Wang, G., Yang, S., Gao, S., Zhao, Y., & Xing, F. (2021). Inhibitory effect of allyl and benzyl isothiocyanates on ochratoxin a producing fungi in grape and maize.
Food
Microbiology,
100.
64
Discussion and feedback