Agrobacterium tumefaciens-MEDIATED IN PLANTA TRANSFORMATION METHOD FOR THE SoSPS1 GENE IN CITRUS PLANTS (Citrus nobilis L.)
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • Vol. 7 No. 1 • September 2019
eISSN: 2655-9994
pISSN: 2303-3371
https://doi.org/10.24843/IJBB.2019.v07.i01.p04
Agrobacterium tumefaciens-MEDIATED IN PLANTA TRANSFORMATION METHOD FOR THE SoSPS1 GENE IN CITRUS PLANTS (Citrus nobilis L.)
Ni Putu Ayu Erninda Oktaviani Suputri1*, Rindang Dwiyani1, Ida Ayu Putri Darmawanti1, and Bambang Sugiharto2
1Faculty of Agriculture, Udayana University, Jalan PB Sudirman Denpasar, Indonesia 2Centre of Development of Advanced Science and Technologhy (CDAST), Jember University, Indonesia
*Corresoponding author: ernindasuputri13@gmail.com
ABSTRACT
The SoSPS1 gene of sugar cane plants previously subjected to Agrobacterium tumefacien-mediated cloning was to be transferred to citrus plants to increase metabolism of sucrose in plant. The T-DNA harbored the SoSPS1 gene under the control of the CaMV 35S promoter from the cauliflower mosaic virus and contained the NPTII gene (kanamycin resistance gene) as a selectable marker for transformant selection. Generally, gene transformation in plants is carried out by tissue culture. However, tissue culture has several disadvantages such as its being time-consuming, its sometimes resulting in somatic mutations and somaclonal variations, and the requirement of sterile conditions in the procedure of gene transfer. In planta transformation is a useful system for those plants that lack tissue culture and regeneration system. The main function of in planta transformation is to recover the advantages of tissue culture as an efficient, quick method, including its ability to produce a large number of transgenic plants and to accumulate a high concentration of total soluble protein in short time. There are two procedures of in planta transformation for the seeds of citrus plants, namely “prick and coat” and “seed tip-cutting and imbibition”. In the prick and coat method, seeds are pricked on their entire surfaces and smeared with a suspension of Agrobacterium tumefaciens. In the seed tip-cutting and imbibition method, on the other hand, seeds are cut at the tip and soaked in a suspension of Agrobacterium tumefaciens. The leaves derived from seeds treatment were taken as samples for DNA extraction and PCR using primers of the NPTII gene (Forward: 5’-GTCATCTCACCTTCCTCCTGCC-3’; Reverse: 5’-GTCGCTTGGTCGGTCATTTCG-3’). This research found that only the seed tip-cutting and imbibition plants amplified along the 550-bp band, while those of the prick and coat method did not. Additionally, the T-DNA was successfully integrated into the genome of the plants treated with the seed tip-cutting and imbibition method but not with the prick and coat.
Keywords: prick and coat, NPTII, cutting, SoSPS1, T-DNA
INTRODUCTION
Orange is one of the commodities classified as high-value fruits important to both the domestic and the world markets, both in fresh and processed forms. Based on Agriculture Statistics (2017), in 2016, the
national citrus consumption per capita increased by 9.76%, while the export volume decreased by 10.5% due to a decrease in the quality of citrus products in Indonesia. Data showed that orange has a high economic value, necessitating improvement of the
quality of the products and the variety in order to achieve high variety, high production, sweet taste, and resistance to biotic and abiotic stresses.
One of the desirable qualities in orange is sweet taste. Improvement of such quality can be performed through insertion of the SoSPS1 gene, a gene that encodes the sucrose phosphate synthase (SPS) protein, cloned from sugar cane plants as a key enzyme in the biosynthesis of sucrose in the plant, resulting in increases in metabolism (photosynthesis) and the level of sucrose in plants (Sugiharto et al., 1997).
Improvement of the plant variety can be carried out conventionally through crossbreeding or in a modern mode through genetic engineering or genetic transformation. Insertion of gene by crossbreeding has the following weaknesses: 1) the process of the gene incorporation is uncontrollable, and the gene expression is unstable and 2) out-crossing becomes impossible. Improvement of variety by genetic transformation can overcome the weaknesses of conventional practices, and the stability of gene transfer in the genomes of plants will be tested through molecular stages. Genetic transformation is the process of introducing a gene from one organism to another directly via particle bombardment (Hansen and Wright, 1999), the use of polyethylene glycol (PEG), and
electroporation (D'Halluin et al., 1992) and indirectly via the help of Agrobacterium tumafaciens (Ishida et al., 1996; Zhao et al., 1998; Negrotto et al., 2000; Frame et al., 2002; Dwiyani et al., 2016;).
Indirect transformation using A. tumefacienss is better than direct transformation because this method is more effective and efficient in application in that, in indirect transformation, the laboratory practice is simple, and the insertion of the gene more stable, than in direct transformation, resulting in higher stability in the gene expression and less genome alteration of the plant transformant (Hansen & Chilton, 1996; Dai et al., 2001; Nishimura et al., 2006; Rahmawati, 2006; Mohammed and Abalaka, 2011).
The utilization of A. tumefaciens in this study was based on the microbe’s ability to infect dicot plants and cause tumors on the trunk (Larebake et al., 1974). A. tumafaciens is also able to transfer recombinant tumor genes into transformation vectors (Dwiyani et al., 2016). Insertion mediated by A. tumafaciens has been successfully carried out on monocotyledonous plants such as rice (Raineri et al., 1990; Park et al., 1996; Komari et al., 1998), maize (Ishida et al., 1996), and banana (May et al., 1995).
Genetic transformation can be performed in vitro or in planta. In vitro transformation generally involves the
introduction of genes into the callus with which plants are to regenerate. The in vitro method comes with some disadvantages: 1) this method is time-consuming; 2) there is a somatic mutation or somaclonal variation in plant cells during the tissue culture; and 3) the process of gene transfer requires a sterile media condition. The in planta transfer method can overcome such disadvantages of the in vitro method. It is essentially simple, efficient, stable, and productive of genes that replicate through a single insertion in the genome (Birch, 1997).
The in planta method involves soaking by which the pollens of flowering plants are inserted without going through the phase of tissue culture (Feldmann and Marks, 1987; Feldmann, 1992). Several types of plants on which in planta genetic transformation has been successfully conducted are Medicago trunttula (Trieu et al. 2000), Arabidopsis thaliana (Bent 2000), apple, pear, tomato, peach, strawberry (Spolaore et al., 2001), and orange (Ahmad and Mirza, 2005).
According to Bhojwani (1979), orange seeds are capable of polyembryonic seeds development in which one seed can develop into more than one embryo. Polyembryony produces zygotic embryo from the fusion of male and female gametes in the nucellus which is formed of an embryo sac. Nucellar embryony is somatic
embryogenesis in which a single cell develops into a group of meristematic cells. Nucellar embryos will grow uniform to the parent into tap roots related to the quality of the fruit which are able to exploit the extreme conditions of the soil and be resistant to specific diseases.
Research on the implementation of this method on orange polyembryonic seeds is needed. The method is innovated from the combination of the prick and soak method (prick and coat) (Sanjaya, 2018) and the imbibition method (seed top-cutting and imbibition). The methods of treatment of the objects of this research are 1) prick and coat (seeds are pricked on the entire surface and smeared with a suspension of Agrobacterium tumefacien), a method inspired by the prick and soak method, and 2) seed top-cutting and imbibition (seeds are cut at the tip and soaked in a suspension of Agrobacterium tumefacien), a method adapted from the imbibition theory.
MATERIALS AND METHODS
The materials research are sucrose phosphate synthase (SoSPS1) gene provided by Prof. Bambang Sugiharto, the Director of the Center for Development of Advanced Sciences and Technology (CDAST), Jember University. The T-DNA harbored the SoSPS1 gene under the control of the CaMV 35S promoter and the NPTII gene, a kanamycin-
resistant gene as a selectable marker for
to December 2019 at the Laboratory of Plant
transformant selection. This T-DNA was constructed in the pKYS plasmid and cloned
to A. tumefacienss strain LBA4404. The experiment was conducted from September
Tissue Culture, the Faculty of Agriculture, Udayana University, and the Laboratory of
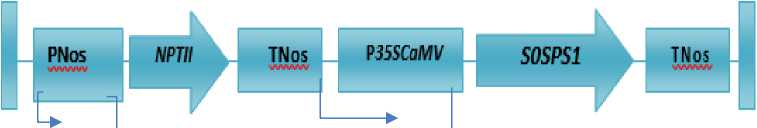
CDAST, Jember University. The T-DNA construct is shown in Fig. 1.
LB RB
600 bp
550 bp
Fig. 1. Agrobacterium tumefacien culture carrying vector with constract pKYS plasmid structure of T-DNA containing SoSPS1 gene and the NPTII gene. Right border, RB; Left border, LB;
Promoter of the nopaline synthase gene, Pnos; Polyadenylation site of the nopaline synthase gene, Tnos; Neomycin Phospotransferase gene, NPTII; 35S promoter of Cauliflower Mosaic Virus, P35SCaMV; Sucrose Phosphate Synthase Gene, S0SPS1 (Source: Sugiharto et al., 1997 in Dwiyani et al., 2019 )
Colonies of bacterium A. tumefaciens were subjected to PCR, grown on a solid LB (Luria-Bertani) medium for reproduction, then moved on a liquid LB medium at 2 mL and added to 50 ppm of antibiotic kanamycin, 100 ppm of rifampin, and 12.5 ppm of gentamicin before being incubated in a shaker at 150 rpm at a temperature of 28 0C for 24 hours until the OD reached 0.6.
After that, each 1 mL of A. tumefaciens culture was placed on 20 mL of liquid LB medium and incubated at a temperature of 24 oC for 24 hours, then transferred to test tubes. The test tubes were covered with sterile aluminum foil and plastic wrap. The test tubes containing cultures of the bacterium were centrifuged at
the speed of 5,000 rpm for 10 minutes. Results setrifugase of solvent with the bacteria to be characterized are precipitated pink (pellet) on the basis of the tube reaction. The supernatant was discarded, and the precipitate re-suspended in an ½ MS medium that already contained 100 ppm of acetosyringone and TWEEN20 to the volume of 200 mL, continuously mixed until homogeneity was reached. The changing of media was carried out within LAFC. The bacterial suspension was then ready to use.
The in planta transformation in this study was performed through three treatments: 1) orange seeds were pierced by a needle and smeared with a suspension of A. tumafacien (prick and coat); 2) orange seeds
were cut at the tip and soaked in a suspension of A. tumafacien for 30 minutes (Bastos et al., 2003; Li et al., 2003) (seed tip-cutting and imbibition); and 3) orange seeds were given no treatment for comparison purpose (control) (Fig.2).
The seeds were planted on growing media (charcoal, husk, soil, and fertilizer) and sterilized at a given temperature to
eliminate pathogens. Rearing was carried out, i.e., watering and weeding every day and adding NPK fertilizer once a week. Plants were harvested at the age of 4 weeks, (a height of 20 cm, 20 sheets of leaves).

Fig. 2. Citrus plant seeds transformation methods: 1. seed tip-cutting and imbibition treatment (a) cutting the tip of the seeds and (b) soaking the seeds in the suspension of A. tumefaciens for 30 minutes, 2. prick and coat treatment (c) pricking the seeds and smearing with A. tumefaciens
The results obtained from the plant DNA isolation were then subjected to a PCR (polymerase chain reaction) process. Samples of plants were added with 7 mL of H2O, 10 mL of master mix, 1 mL of NPTII forward primer (5'-GTCATCTCACCTTGCTCCTGCC-3'), and 1 mL of NPTII reverse primer (5'-GTCGCTTGGTCGGTCATTTC-3') in eppendorf. PCR was performed through the pre-denaturation stage at 95 ºC for 3 minutes,
the denaturation stage at 95 ºC for 30 seconds, the annealing stage at 59 ºC for 30 seconds, the extension stage at 72 ºC for 1 minute, and the final extension at 72 ºC for 5 minutes, 35 times. From PCR, the samples were then inserted into wells of agarose gel and run for 25 minutes at 100 volts. The results obtained were then visualized in a UV cabinet.
Agrobacterium tumefaciens-MEDIATED In Planta TRANSFORMATION METHOD FOR THE SoSPS1 GENE IN CITRUS PLANTS (Citrus nobilis L.)
RESULTS AND DISCUSSION
Before we did the transformation, we confirmed the gene of interest inside the Agrobaterium. Ten (10) single colonies of Agrobacterium were tested. The results can be seen in Fig. 3. In this image, the gene is
P 1 2 3 4 5
Ni Putu Ayu Erninda Oktaviani Suputri, Rindang Dwiyani, Ida Ayu Putri Darmawanti, and Bambang Sugiharto
still harbored by all of the single colonies tested. They are indicated by 550-bp bands amplified using NPTII primers (Forward: 5’-GTCATCTCACCTTCCTCCTGCC-3’;
Reverse: 5’
GTCGCTTGGTCGGTCATTTCG-3’).
6 7 8 9 10 M
550 bp
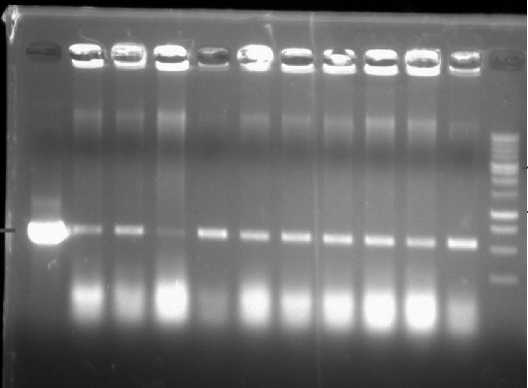
Fig. 3. The gene confirmation in the A. tumefaciens using NPTII primers. P = plasmid; 1–10 = single colonies of A. tumefacienss; M = DNA marker of 1 kb DNA ladder.
3000 bp
1000 bp
To describe the success of the transformation method, calculation of the percent efficiency of the citrus plants transformation treatments was performed. Of the 30 seeds in each treatment with A. tumefaciens and control, the seeds found to be transformation-positive amounted 16.7%
in the seed tip-cutting and imbibition treatment, 0% in the prick and coat treatment, and 0% in the control. The data of the percent efficiency of the plant transformation are shown in Table 1.
Table. 1. (%) Efficiency of Plant Transformation
|
Treatment |
Σ Seeds treated |
∑ Seeds germinated |
∑Transformative putative plants |
(%) Efficiency of Plant Transformation |
|
Prick and Coat |
30 |
1 |
- |
0% |
|
Seed Tip- |
16.7% | |||
|
Cutting and |
30 |
5 |
5 | |
|
Imbibition | ||||
|
Control |
30 |
30 |
- |
0% |
Isolation of DNA used the modified mini-prep method by Zang and Stewart (2000). Reading was performed on the three treatments samples after electrophoresis, and the results of the molecular analysis are shown in Fig. 4. PCR analysis was performed by using the NPTII primer for the genes with an amplification estimated at the 550-bp band in the treatment of seed tipcutting and imbibition (PR). Fig. 4 shows the results of the molecular analysis of seven samples: the control (K), candidate transformants in the seed tip-cutting and imbibition treatment replicate 1 (PR1),
candidate transformants in the seed tipcutting and imbibition treatment replicate 2 (PR2), candidate transformants in the seed tip-cutting and imbibition treatment replicate 3 (PR3), candidate transformants in the seed tip-cutting and imbibition treatment replicate 4 (PR4), candidate transformants in the seed tip-cutting and imbibition treatment replicate 5 (PR5), and candidate plant transformants in the prick and soak treatment replicate 2 (TL2). The analysis was carried out in two stages: 1) to compare between treatments and 2) to confirm methods of the treatments and replications.
600 bp
500 bp
550 bp f
600 bp
500 bp
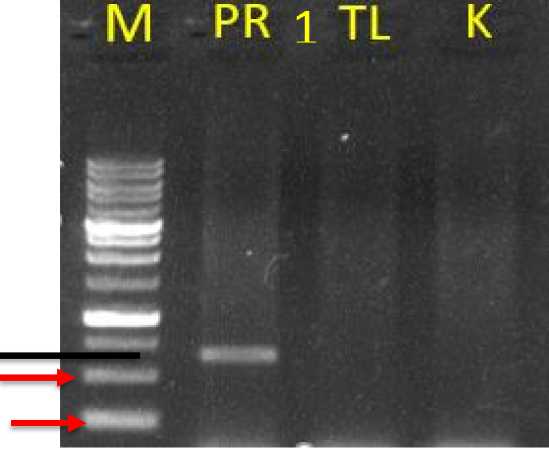
Fig. 4. A PCR visualization of transgenic candidate citrus plants, M (Marker), PR 1 (Seed tip-cutting and imbibition repeat 1), TL (Prick and Coat), K (Control)
550 bp
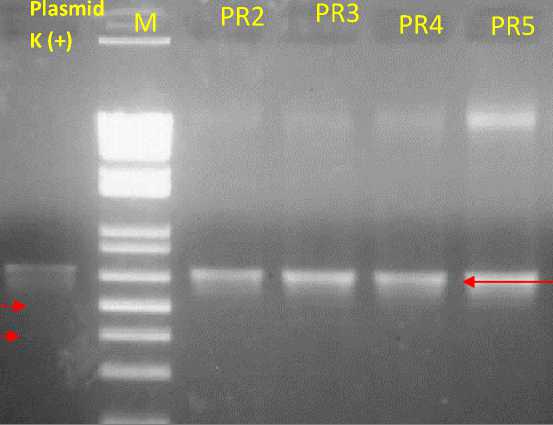
Fig. 4. B PCR visualization of transgenic candidate citrus plants between replications, M (Marker), pKYS-SoSPS1 plasmid as positive control, PR2 (Seed tip-cutting and imbibition repeat 2), PR3 (Seed tip-cutting and imbibition repeat 3), PR4 (Seed tip-cutting and imbibition repeat 4) and PR5 (Seed tip-cutting and imbibition repeat 5)
The transformation method used was the in planta transformation. In planta transformation is the process of direct gene transfer to parts of plants that undergo
meristematic phases, one of which is the seed (somatic embryogenesis). This is because the gene replication in the plant genome takes place in a large amount, making the gene
stable and reproducible. In planta transformation is more efficient than in vitro method. It is considered simple and easy to do because it targets ex vitro plants, or plants that are not derived from tissue culture. In planta transformation offers a solution to the weaknesses of the in vitro method: the process requires a long period of time and more skilled labor, and it may generate somaclonal variation (Kalbande and Patil, 2016).
The in planta method performed on orange seeds in the present research was modified from the prick and soak method by Sanjaya (2018) in which seeds were pricked and coated with a suspension of A. tumefaciens (TL). Besides, a method adapted from the seed imbibition theory (Salisbury and Ross, 1992) called seed tip-cutting and imbibition (PR) was also used.
The results of the use of the in planta method are shown in Table 1, and the results of the PCR analysis in Fig. 4. From the incorporation of A. tumefaciens on 60 seeds in the treatments and control, it was found that the transformation in the seed tip-cutting and imbibition (PR) treatment amounted 16.7%, in the prick and coat treatment (TL) 0%, and the control 0%. The seed tip-cutting and imbibition (PR) generated a good level of efficiency but improvement is necessary by several factors: 1) soaking of orange seeds in suspension and 2) temperature of the citrus
plant seeds germination (Lester, 2019). The seed tip-cutting and imbibition method (PR) integrates the NPTII gene into the plant genome. Based on the visualization of the PCR results, amplification took place along the 550-bp band (Fig. 4. A), and analysis continued between five replicates (Fig. 4. B), generating the same results.
The suspension of A. tumefaciens consists of nutritious MS medium, colonies of A. tumefaciens, and acetosyringone. According to Salisbury and Ross (1992), high-concentration A. tumefaciens suspension is able to go into low-concentration seed cells. Seed imbibition is the spontaneous absorption of liquid by porous, dry material. In polyembryony, the highly porous orange seeds were infiltrated with the suspension through the passive transport of osmosis, with the structure of the pores affecting the rate of the suspension absorption. (Jean et al., 2018).
The factor for the transformation success in this study was the use of TWEEN20, transformational explants, and acetosyringone (AS). Clough and Bent (1998) stated that the administration of surfactant at a low concentration on the immersion medium by the floral-dip method can increase the number of transformants obtained in the plant Arabidopsis thaliana. TWEEN20 is one of surfactant types which serves to lower the tension of the surface of
the cell, reducing the hydrophobicity of the plant and bacterium cells and facilitating the attachment of A. tumefaciens in the plant cells (Miller, 2013).
The explants also influenced the success of the transformation. It was associated with the physiological process the plant organs were engaged in. Endogenous phenol compounds in seeds function as active agents that stimulate germination in anaerobic conditions. Total phenol varies according to the type or variety. Phenol compounds in seeds will naturally be active in the imbibition process (Pramono, 2017). They have a major influence and role in activating virgen to release T-DNA from plasmids and deliver T-DNA to plant cells to be integrated with plant genomes. Additionally, the increase in the concentration of synthetic acetosyringone (AS) will influence efficiency and success and shorten the time of immersion in eggplant seeds (Chumakov, 2007).
Based on the data in Table 1, the seed tip-cutting and imbibition method (PR) had a percentage of transformative efficiency of 16.7%, and this success rate can be increased by internal factors of orange seeds (the physiological state of recalcitrant seeds): the imbibition process time. Citrus plants seeds which have a porous structure require only a short period of time. Soaking for 30 minutes is less efficient, causing hypersensitivity to
the cell seeds. According to Jones and Dangl (2006), hypersensitive reactions are generally active during attacks of pathogens in molecular plant cells. The stages begin with the plant recognizing the incoming pathogens and starting to activate the resistance system. Meanwhile, the pathogens successfully deploy effector that are virulent. Effectors that are recognized by the plant's immune system send signals to nucleotides. The nucleotides resist pathogens by means of lethal cells (hypersensitive reaction).
The second factor was seed germination temperature. In this study the seeds were germinated at medium humidity, giving the seeds a stress and leading to a decrease in metabolism. The decrease in the seed quality was indicated by an increase in the fat content. Fungi attack the seeds during germination at normal humidity, matter is the result percentace growing from citrus plants is low and also affects the percentage of putative efficiency of transformed plants (Worang et al., 2008). Meanwhile, a high content of fatty acids indicates that the process of respiration was high, causing the seeds to lose energy for germination. Germination of seeds would run well at an optimum temperature of 14 ºC, stored under a dry, cold condition. This is because the quality of the seeds would be optimal under such conditions, thereby increasing the speed of growth. (Pradhan and Badola, 2012).
The inhibiting factors in the transformation by the prick and coat method (TL) included the puncturing of the seeds and the use of explants. The seeds used was recalcitrant ones, which have high levels of water to maintain the vigor and viability of the seeds. Pricking treatment would reduce the quality of the orange seeds. The biochemical indicators (fat, protein, carbohydrate) in the seeds which experienced a decline in the quality showed a change in the activity of the enzyme, a change in the rate of respiration, a change in the food reserves, a change in the membrane, a damage to chromosomes, and accumulation of toxic materials (Tatipata, 2008). The pricking process would decrease the water level and germination power of the seeds, so the fat content in the seeds tended to increase and the carbohydrate and protein levels
decrease, causing the seeds to lose energy for germination (Yuniarti et al., 2008).
The puncturing of the seeds will cause a state of collapse to the cells as shown in Fig. 5. The seed sell collapse during the incorporation of the suspension of A. tumefaciens would inhibit the production of ATP in the cells by poisoning of the mitochondria. So genes that throught to insertion not be replicated and the gene is not amplified in the genome of plants. Potential damage to cell membranes due to puncture and infection with A. tumefaciens indicates that the supply of ATP influences the success of the gene replication process. Then, based on the rigidity of the cells, it is important to carry out genetic transformation and health maintenance of the citrus seeds (Salisbury and Ross, 1992).
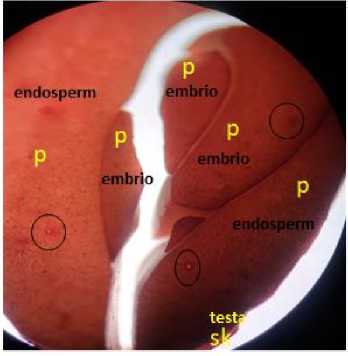
Fig. 5. Microscope 10 X
The anatomy of the seed is comprised of testa, endosperm, and embryo tissues; the testa tissue consists of sclereid cells (SK), while the endosperm and embryo tissues consist of parenchyma cells (P). The circles mark the holes due to puncturing. (private document)
CONCLUSIONS
The in planta transformation by prick and coat method and seed tip-cutting and imbibition method on citrus seeds could integrate genes into the plant genome and produce transformant orange crops. Seed oranges are seeds are rekasiltran were not able to do the method with stabbing.
REFERENCES
Ahmad, M., B. Mirza. (2005). An efficient protocol for transient transformation of intact fruit and transgene expression in Citrus. Mol Mol Plant . 7: 114-135
Bastos, WA., AAM Almeida, BMJ Francisco,. Filho, A. Mendes, Pavan. (2003). Agrobacterium - Mediated Transformation of Citrus sinensis Epicotil Segments. Scienta Agricola . 60 (7) : 23-29
Bent, AF. (2000). Arabidopsis in planta Transformation. Uses, Mechanisms, and Prospects for Transformation of Other Species. The USA. American Society of Plant Physiologists. 124 (5) : 1540-1547
Berjak, P., N. W. Pammenter. (2013).
Implications of the lack of desiccation tolerance in recalcitrant seeds. Frontiers in Plant Science, 4 ( 2 ) : 1 – 9.
Bhojwani, SS and SP Bhatnagar. (1979). The Embriology of Angiosperm. New Delhi: Vikas Publishing House : 2229
Birch, RG. (1997). Plant transformation -problems and strategies for pratical applications. Annu. Rev. Plant Physiol. Plant. Mol. Biol 48 (7 ) : 297-326
Chumakov. R. (2007). Functional Organization of the Plant Nucleus. Plant Cell Monograph Springer Book : 166-168
Clough, SJ, AF Bent. (1998). Floral dip: a simplified method for Agrobacterium mediated transformation of
Arabdopsis thaliana. Plant J. 16 (4): 735 - 743.
Dai, S., P. Zheng, P. Marmey, S. Zhang, W. Tian, S. Chen, R. N. Beachy, C. Fauquet. (2011). Comperative analysis of transgenic rice plants obtained by Agrobacterium- mediated transformation and particle
bombardment. Mol. Breed 7 (2): 2533.
D'Halluin, K., E. Bonne, M. Bossut, M. De Beuckleer, and J. Leemans. (1992). Transgenic maize plants by tissue electroporation. Plant Cell 4: 14951505.
Dwiyani, R., H. Yuswanti,, IDA Darmawti , INA Mayadewi. (2016). Genetic Transformation in Plants through Agrobacterium tumafciens. Denpasar: Swata Nulus
Dwiyani, R. H. Yuswanti, Y. Fitriani dan B. Sugiharto. (2019). In Planta Transformation Method Mediated with Agrobacterium tumefaciens for T-DNA Transfer in Table Grape (Vitris vinifera L.) 6 (2): 95 - 105
Feldmann, KA. (1992). T-DNA insertion
mutagenesis in Arabidopis: seed infection transformation. In C Koncz, NH Chua, J Schell, eds, Methods in Arabidopsis Research. World Scientific, Singapore: 274–289
Feldmann, KA Marks, MD. (1987). Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue c mL ture approach. Mole Genet 208: 1–9
Frame, BR, H. Shou, RK Chikwamba, Z. Zhang, C. Xiang, TM. Fonger , S.
Ellen, K. Pegg , B. Li, DS Nettleton, D. Pei, and K. Wang. (2002). Agrobacterium tumefaciens-Mediated Transformation of Maize Embryos Using a Standard Binary Vector System. Plant Physiol. 129 (3) : 1322.
Hansen, G., and MS Wright. (1999). Recent advances in the transformation of plants. Trends in Plant Science. 4 (6), 226-231.
Hansen, G., MD Chilton, (1996). "
Agroholistic " transformation of plant cells: Integrated of T-stands generated in planta. Porc. Natl.Acad.Sci . USA 93: 14978 - 14983
Ishida, Y., H. Saito, S. Ohta, Y. Hiei, T. Komari, T. Kumashiro. (1996). High efficiency transformation of Maize ( Zea Mays L.) mediated by Agrobacterium tumefaciens. Nature Biotechnol 14: 745-750.
Jean, F, Louf, Y. Zheng, A. Kumar, T. Bohr, C. Gundlach, J. Harholt, HF Poulsen, KH Jansen. (2018). Imbibition in Plant Seeds. Research Laboratory: 16
Jones, JD and JL Dangl. (2006). The Plant Immune System. Nature, 444: 323329.
Kalbande, BB and AS Patil. (2016). Plant Tissue Culture Independent
Agrobacterium tumefaciens Mediated In-Planta Transformation Strategy for Upland Cotton (Gossypium
hirsutum). Journal of Genetic Engineering and Biotechnology, 14: 9-18.
Komari, TY Hiei, Y. Saito, N. Murai, T. Kumashiro. (1996). Vector carrying two separate T-DNAs for cotransformation of higher plants mediated by Agrobacterium
tumefaciens and segregation of transformation free from selection markers. Plant J. 10: 165-174.
May, JH, Lawton, M. Robert. (1995). Ecological, Ecosystem, Biological Extensions and Ecological Crisis. 233
Miller, PD. (2013). Method for Improved Transformation Using
Agrobacterium. United States Patent Application Publication. US2013 /
0157369A1: 1-14.
Mohammed, and Abalaka. (2011). Agrobacterium Transformation: A Boost to Agric µl tural Biotechnology. Journal of Medical Genetics s and Genomics. 3 (8): 126130.
Negrotto, D., M. Jolley, S. Beer, AR Wench, G. Hansen. (2000). The use of phosphomannose- isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep. 19: 798803.
Nishimura, A., I. Aichi, M. Matsuoka. (2006). A Protocol for Agrobacterium - mediated Transformation in Rice. Nature Protocols. Vol. 1
Park, MX Wu, K. Golden, JD Axelford, R. Bordmer. (1996). The wingless signaling pathway is directly involved in Drophosila heart development. Dev Biol 177: 104-116
Pradhan, BK, & HK Badola. (2012). Effects of storage conditions and storage periods on seed germination in eleven populations of Swertia chirayita: A critically endangered medicinal herb in the Himalayas. The Scientific World Journal. 10 (2): 1–9
Pramono, E. (2010). Ethanol, Metabolism, and Seed Decline. Agronomy affairs, Faculty of Agriculture, University of Lampung
Rahmawati S. (2006). Status of development of improved genetic traits of rice using Agrobacterium transformation. AgroBiogen 2 (1): 36-44.
Raineri, DM Bottino, P. Gordon. (1990).
Agrobacterium mediated
transformation of rice (Oryza sativa) Bio / technology. 8: 33
Salisbury, PB, C. W. Ross. (1992). Plant Physiology. California (US):
Wadsworth Pub.Com.belmont . p. 682.
Spolaore, S. L. Trainotti, G. Casadoro. (2001). A simple protocol for transient gene expression in ripe fleshy fruit mediated by
Agrobacterium. J Exp Bot .; 52: 845 -50.
Sugiharto, B., H. Sakakibara, Sumadi, and T. Sugiyama. (1997). Differential Expression of Two Genes for Sucrose Phosphate Synthase in Sugarcane: Molec µl ar Cloning of the cDNAs and Comparative Analysis of Gene Expression. Plant Cell Physiol. 38: 961-965.
Tatipata, A. (2008). Effect of initial water content, packaging and shelf life on membrane proteins in mitochondrial soybean seeds . Agronomy Bulletin 36 (1): 8-16.
Trieu, AT. SH Burleigh, Kardailsky, Mendoza., WK Versaw, LA Blaylock, (2000). Transformation of Medicago truncat mL of a via infiltration of seedlings or flowering plants with Agrobacterium. Plant J.22 (2) : 531–41.
Sanjaya, IPW. (2018). In planta Transformation in Tomato Plants (Lycopersicon esc µl entum Mill.) Through Agrobacterium tumefaciens . Description . Udayana University. 3032
Worang, RL, QS. Dharmaputra, R.Syarief ,, Miftahudin. (2008). The quality of physic nut (L.) seeds packed in plastic
Jatropha curcas material during storage. , 15 (1) : 25 - 36.
Yuniarti, N., D. Syamsuwida,, A. Aminah. (2008). Impact of drying to change the physiology and biochemistry seeds of neem (A. Jusss). Azadirachta indica Bulletin of Research and Development Perhutani, 11 (1) : 728735.
Zang, J., and J. McD. Stewart. (2000). Economical and Rapid Method for Extracting Cotton Genomic DNA. The Journal of Cotton Science, 4: 193-201.
Zhao, ZY, W. Gu, T. Cai, LA Tagliani, DA Hondred, D. Bond, S. Krell, ML Rudert , WB Bruce, DA Pierce. (1998). Molec µl ar analysis of T0 plants transformed by Agrobacterium and comparison of Agrobacterium-mediated transformation with bombardment transformation in Maize. Maize Genet. Coop Newslett . 72: 34-37.
44 • FACULTY OF AGRICULTURE, UDAYANA UNIVERSITY
Discussion and feedback