IN-PLANTA TRANSFORMATION METHOD MEDIATED WITH Agrobacterium tumefaciens FOR T-DNA TRANSFER IN TABLE GRAPE (Vitis vinifera L.)
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • Vol. 6 No. 2 • April 2019 eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2019.v06.i02.p02
IN-PLANTA TRANSFORMATION METHOD MEDIATED WITH Agrobacterium tumefaciens FOR T-DNA TRANSFER IN TABLE GRAPE (Vitis vinifera L.)
Rindang Dwiyani1*, Hestin Yuswanti1, Yuyun Fitriani1, and Bambang Sugiharto2 1Faculty of Agriculture, Udayana University, Jalan PB Sudirman Denpasar, Indonesia
-
2Centre Development of Advanced Sciences and Technology, Jember University, Jl. Kalimantan No.
-
37, Kampus Tegalboto Jember – Jawa Timur, Indonesia *Corresponding author: rindangdwiyani@unud.ac.id
ABSTRACT
The aim of the research is to investigate a simple method of in planta transformation method for T-DNA transfer in table grape. The T-DNA harbored the S0SPS1 gene under the control of promoter of the 35S CaMV from the Cauliflower Mosaic Virus and contained the NPTII gene, a kanamycin-resistant gene as a selectable marker for transformant selection. Sixmonth plants originated from cuttings were used as target plants. We explored two methods of in planta transformation, namely ”dipping” and “sweeping”. For both methods, the leaves of the target plants were removed and those of shoots without leaves were used as the target of transformation. In the “dipping method”, those shoots were dipped with the agrobacterial suspension for 60 seconds. However, for the “sweeping method”, the scars (the spots where leaves were removed) were swept with agrobacterial suspension using a cotton bud. Those treated non-leafy-shoots (from both methods) then were grown to be leafy shoots. Those leafy shoots then were cut and transplanted into the soil and grown to be a whole plant. The leaves of those plants then were taken as samples for DNA extraction and PCR using primers of NPTII gene (Forward: 5’-GTCATCTCACCTTCCTCCTGCC-3’; Reverse: 5’
GTCGCTTGGTCGGTCATTTCG-3’) with expected amplified band of 550 bp. We found that only the “sweeping method” plants amplified the 550 bp bands, while those of the “dipping method” did not. We suggest that the T-DNA was successfully integrated into the genome of plants treated with the “sweeping method” but not with the “dipping method”. Leaf sugar content (oBrix) of PCR-positive vines was higher than those of the wild-type vines, ensuring the integration of the T-DNA into the plant genome.
Keywords: dipping, NPTII, sweeping, S0SPS1, T-DNA
INTRODUCTION
Genetic transformation is an efficient way to improve the characters of plants. The most common methods for the introduction of DNA into plant cells use Agrobacterium tumefaciens or rapidly propelled tungsten microprojectiles that have been coated with DNA (Birch 1997; Hansen & Wright 1999). However, we suggest that the cheapest and
simplest method is using A. tumefaciens. Genetic transformation method using A. tumefaciens based on tissue culture (in vitro) methods has been reported elsewhere for some plant species such as a medicinal plant Artemisia aucheri Boiss (Sharafi et al. 2014), Pisum sativum (Svabova et al. 2005), tomato (El-Siddig et al. 2009), Patchouli [Pogostemon cablin (Blanco) Benth] ( Paul
et al. 2012), Orchid (Dwiyani et al. 2010; Liau et al. 2003; Semiarti et al. 2007). However, those tissue culture based-methods are suggested to be time consuming and lead to soma clonal variation that affect both qualitative and quantitative characters of the plants (Labra et al. 2004). The direct transformation method without any tissue culture steps is termed as in-planta transformation (Feldmann & Marks 1987). The production of a large number of uniform plants in a short time, less labor efforts, and minimal reagents requirements are some of main the advantages of the in-planta transformation system (Bent 2000). In addition, tissue culture-based transformation methods require carefulness to maintain sterile condition, a common difficulty in the tissue culture work.
We explored in-planta transformation of a table grape in order to provide a simple method of transformation that may be easier to be done compared to the in-vitro method. In-planta transformation method has been done for Arabidopsis by applying Agrobacterium to the Arabidopsis seeds as the target of transformation (Feldmann 1992; Feldmann & Marks 1987) and a method of “clip ‘n squirt” using inflorescences as targeted cells (Chang et al. 1994; Katavic et al. 1994). In-planta transformation method mediated with Agrobacterium was also reported for Brassica napus L. (Li et al.
2010), wheat (Razzaq et al. 2011), pommelo (Citrus maxima) (Zhanga et al. 2017) and Phalaenopsis orchid (Semiarti et.al. 2014), but there is a lack of report for table grape. The current research explored two methods using A. tumefaciens with non-leafy shoots (shoots whose leaves were removed) as targeted cells, namely ‘dipping method’ and ‘sweeping method’. The aim of the research was to investigate a simple method of inplanta transformation for the table grape. The current method would be easier to be done and may be adopted for other species.
MATERIALS AND METHODS
We used a Sucrose Phospate Syntase (S0SPS1) gene that was obtained from Prof. Bambang Sugiharto, the Director of the Center Development of Advanced Sciences and Technology (CDAST), Jember University. The T-DNA harbored the S0SPS1 gene under the control of the 35S CaMV promoter and the NPTII gene, a kanamycin resistant gene as a selectable marker for transformant selection. This T-DNA was constructed in pKYS plasmid and it was cloned to A. tumefaciens strain LBA4404. The experiment was conducted from January to October 2017 at the Laboratory of Plant Tissue Culture, the Faculty of Agriculture, Udayana University and the Laboratory of CDAST, Jember
University. The T-DNA construct is shown in Fig. 1.
LB RB
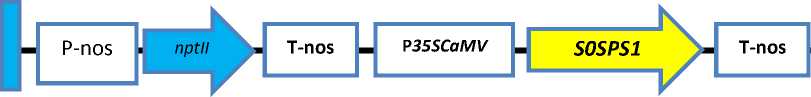
Fig.1. The structure of T-DNA in pKYS plasmid harbored nptII gene and S0SPS1 gene. Right border, RB; Left border, LB; Promoter of the nopaline synthase gene, Pnos;
Polyadenylation site of the nopaline synthase gene, Tnos; Neomycin Phospotransferase gene, nptII; 35S promoter of Cauliflower Mosaic Virus, P35SCaMV; Sucrose Phosphate Synthase Gene, S0SPS1 (Source: Sugiharto et al. 1997)
The preparation of Agrobacterium suspension was done using a method mentioned in Dwiyani (2012). A single colony of A.tumefaciens that harbored the gene was cultured on 2 ml of liquid YEP Medium which contained 50 ppm kanamycin, 100 ppm gentamycin and 100 ppm ryfamphycin for 24 hours until the 0.6 Optical Density (OD) was reached. Then, 1 ml of the bacterial culture was added with the same medium until the volume of 20 ml for subsequent culture in the shaker until the OD of 0.6 was accomplished. The culture then was centrifuged with 1000 rpm for 5 minutes. The supernatant was removed and replaced by ½ MS liquid medium. The agrobacterial suspension was then added with 100 ppm acetosyringone and 2µL tween and was used for in-planta transformation. The suspension was diluted ten times with ½ MS liquid medium before it was used.
Six-month of table grape (Vitis vinifera L. var. Alphonse lavallee) plants originated from cuttings were used as target plants. We selected forty (40) healthy shoots from twenty (20) plants.. The leaves of the shoots were trimmed and removed. The purpose of removing the leaves was to allow buds in the leaf axil to grow to be new shoots. Besides that, the scars (spots where the leaves were removed) provided channels for Agrobacterium to enter the plant cells. The non-leafy shoots were then used as the target of the in-planta transformation (Fig. 2). We explored two methods in the current research, namely the “dipping method” and “sweeping method”. In the dipping method, the non-leafy shoots were dipped with the Agrobacterial suspension for 120 seconds (Fig. 3). However, for the “sweeping method”, the scars were swept with the Agrobacterial suspension using a cotton bud (Fig. 4). After 7 days of treatment, leaves
emerged and grew to be new shoots. At 14 grew to be a whole plant in the two (2)
days of treatment, the new shoots were cut months after the treatment (Fig. 5).
and transplanted in-to the soil. Those shoots

Fig. 2. The non-leafy shoots (red arrows) as the target of in planta transformation

Fig. 3. Dipping of the non-leafy shoots on the “Dipping method”

Fig. 4. Sweeping the scars (spots where leaves were removed) with Agrobacterial suspension using a cotton bud on the “sweeping method”

Fig. 5. Young plants originated from the cutting of the new shoots after treated with agrobacterial suspension; A= young plant from the dipping method; B=young plant from the sweeping method; bar=3cm
RESULTS AND DISCUSSION
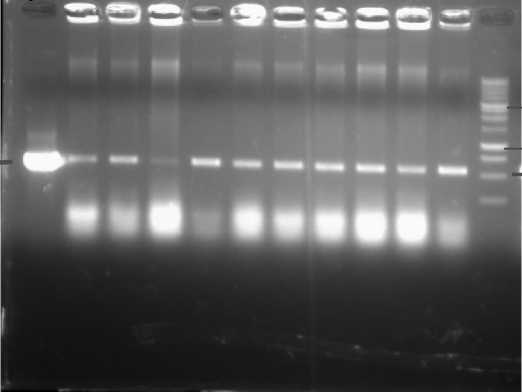
Before we did the transformation, we confirmed the gene of interest inside the Agrobaterium. Ten (10) single colonies of Agrobacterium were tested. The results can be seen in Fig. 6. In this image, the gene is still harbored by all of the single colonies
tested. They are indicated by 550bp length of band amplified using NPTII primers (Forward: 5’-
GTCATCTCACCTTCCTCCTGCC-3’;
Reverse: 5’
GTCGCTTGGTCGGTCATTTCG-3’).
1 2 3 4 5 6 7 8 9 10 M
550 bp
3000 bp
1000 bp
500 bp
Fig. 6. The gene confirmation in the A. tumefaciens using NPTII primers. P = plasmid; 1-10=single colonies of A. tumefaciens; M=DNA marker of 1 kb DNA ladder.
We used 40 shoots from 20 table grape plants for the transformation. Twenty (20) shoots were treated with the dipping method and the other twenty were treated with the sweeping method. However, after the new shoots were cut and transplanted; only nine were successfully grown to be young plants, five were from the dipping method and four were from the sweeping method. At the first analysis, we used only four plants as samples for the PCR analysis, i.e. 1 control plant, 2 plants originated from the shoots treated with the dipping method, and 1 plant originated from the shoots treated with the sweeping method. Without the selection process with kanamycin, the leaves of those plants were taken as samples for DNA extraction (Doyle & Doyle 1990) and
then was PCR analyzed. In the current research, we verified the integration of T-DNA containing NPTII gene in the table grape using primers for NPTII gene (Forward: 5’-GTCATCTCACCTTCCTCCTGCC-3’;
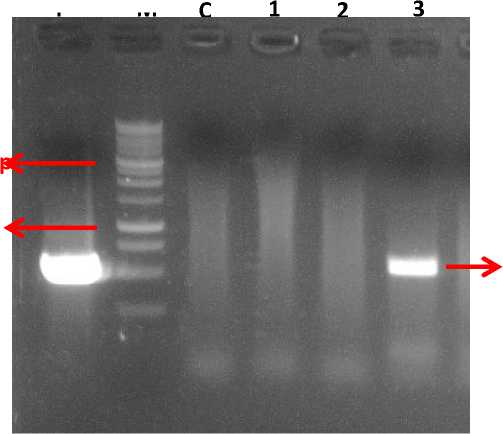
Reverse: 5’ GTCGCTTGGTCGGTCATTTCG-3’) with expected amplified band of 550 bp. Fig. 7 shows the electrophoresis graph of the PCR results. We found that only the plant originated from the shoots of the sweeping method amplified the 550 bp band, indicating that the gene may integrated into the genome of the plant originated from the sweeping method, but did not occur with plants from the dipping method.
P
M
3000 b
1000
550
Fig. 7. The electrophoresis graph of PCR analysis using the NPTII primers.
P=plasmid; M= DNA marker of 1 kb DNA ladder; C= control plant; 1-2 = plants of the dipping method; 3 = plant of the sweeping method
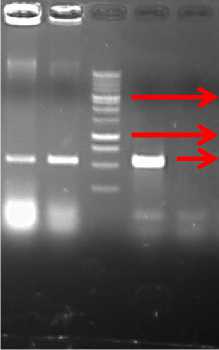
To ensure the results, then we did PCR for the other putative transformed plants. Two plants were from the sweeping method and one was from the dipping method. The PCR result is shown in Fig. 8.
The second PCR analysis again confirmed that the transgene was inserted into the plant genome of the sweeping method plant, but it did not occur with those of the dipping method.
1 2 M P 3
3000 bp
1000 bp
550 bp
Fig. 8. The electrophoresis graph of the second PCR analysis for other transformed plants using primers of the NPTII gene. 1-2 = plants of the sweeping method; 3= plant of the dipping method; P= plasmid; M= DNA marker of 1 kb DNA ladder.
We checked also the sucrose content of leaves of the PCR-positive vines and wildtype vines with hand-refractometer as the S0SPS1 gene was included in the T-DNA construct. The leaves were crushed with
mortar, and 1 µL of the extract was diluted 5 times by adding with 4 µL of distilled water and then measured with hand-refractometer. The result is in the Table 1.
Table 1. Leaf Sugar Content (oBrix) of PCR-PositiveVines and Wild Type vines (measured with Hand–refractometer)
Treatment
Wild Type Vines
Sugar Content (oBrix)
Vine 1 : 2.8
Vine 2 : 2.0
Vine 3 : 2.8
Vine 4 : 2.9
PCR-Positive Vines
Vine 1 : 5.8
Vine 2 : 5.4
Vine 3 : 5.2
Vine 4 : 5.8
Vine 5 : 5.1
Vine 6 : 5.3
The data in the Table 1 suggested that the S0SPS1 gene might be inserted in to the plant genome and was functionally worked on the putative vines. As we know that the Sucrose Phosphate Synthase is a key enzyme for sucrose biosynthesis in plants (Bruneau et al., 1991).
When scars (spots where leaves were removed) were swept with the Agrobacterium solution, the Agrobacterium might enter the plant cells, and the T-DNA was integrated into the plant genome. Thus the new shoot emerging from the node carried the gene of interest. The new shoot was cut and grown to be a whole plant, and we suggest it as a candidate of transformant. All transformants produced from this method (4 plants) confirmed the existence of the NPTII gene. On the contrary, it did not occur in the dipping method, event when the scars were dipped for 120 seconds. The T-DNA failed to integrate into the plant genome, and the method did not produce any transformant. However, we need further research to increase immersion time to allow the chance of gene integration. We also need to confirm the cimeras and ensure that the gene was integrated in the whole plant.
Transferred DNA (T-DNA) enters the plant as a single stranded molecule (Stachel et al. 1986; Tinland et al. 1994; Yusibov et al. 1994) that may eventually integrate into the nuclear genome. Although T-DNA
integration is random (Kim et al. 2007), the mechanism of integration remains unclear (Park et al. 2015). However, irradiated protoplasts show a higher DNA integration frequency than do non-irradiated protoplasts (Kohler et al. 1989), suggesting that double stranded (ds)DNA damage sites could be targets of T-DNA integration (Park et al. 2015). Indeed, T-DNA molecules preferentially integrate into dsDNA break sites (Chilton and Que, 2003; Salomon and Puchta, 1998; Tzfira et al. 2003). In the current research, the touching of a cotton bud into the shoot scars in the sweeping method may produce dsDNA damage sites, providing a chance of T-DNA to integrate into the plant genome.
The transformation method for table grape had been regenerated in vitro (Dabauza & Velasco 2012; Mezzetti et al. 2005), however, the in planta method for table grape has been rarely studied. Fujita et al. (2009) did successful in planta transformation on table grape cultivars (Chardonnay and Cabernet Sauvignon). They used dormant buds on cuttings as the target of the transformation. The dormant buds were pricked four times with a needle and dipped into the A. tumefaciens solution overnight. It was different from the current method. Although we had not done the southern blot yet for gene confirmation, however, this preliminary research suggested
that the current method of “sweeping method” was a novel simple method of in planta transformation for T-DNA transfer in table grape, a similar protocol has not been reported elsewhere. The findings may guide future efforts to improve the transformation of other plant species.
CONCLUSIONS
The current preliminary research of in planta transformation method for T-DNA transfer in table grape was suggested as a novel simple method in transferring the T-DNA into the plant genome. The method named “sweeping method”. It was done by sweeping the scars of shoots without leaves (the non-leafy shoots) with agrobacterial suspension. The production of transformed plant was done by cutting the new emerging shoots from the swept scars and those were then grown to be new plants.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support provided by the Udayana University (Denpasar, Bali, Indonesia) through Competitive Grant Project (Hibah Unggulan Udayana, 2017). The authors also acknowledge Prof. Bambang Sugiharto from the Centre Development of Advanced Sciences and Technology (CDAST), Jember University, for facilitating the molecular research.
REFERENCES
Bent, A. F. (2000). Arabidopsis in Planta Transformation. Uses, Mechanisms, and Prospects for Transformation of Other Species. Plant Physiol. 124 : 1540-1547
Birch, R. G. (1997). Plant transformation: problems and strategies for practical application. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 297–326
Bruneau, J. M., Worrell, A. C., Cambou, B., Lando, D., and Voelker T. A. (1991). Sucrose Phosphate Synthase, a Key Enzyme for Sucrose Biosynthesis in Plants. Plant Physiol. 96(2): 473–478
Chang, S. S., Park, S. K., Kim, B. C., Kang, B. J., Kim, D. U., and Nam, H. G. (1994). Stable genetic transformation of Arabidopsis thaliana by
Agrobacterium inoculation in planta. Plant J. 5: 551–558
Chilton, M. D., and Que, Q. (2003).
Targeted integration of T-DNA into the tobacco genome at double
stranded breaks: new insights on the mechanism of T-DNA integration. Plant Physiol. 133:956–965.
Dabauza, M., and Velasco, L. (2012). Development of highly efficient
genetic transformation protocols for table grape Sugraone and Crimson Seedless. Methods Mol. Biol. 847: 227-235
Doyle, J. J., and Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus 12: 13-15.
Dwiyani, R., Purwantoro, A., Indrianto, A., and Semiarti, E. (2010). Improvement of Genetic Transformation Efficiency in Vanda tricolor Orchid Using Acetosyringone. Annales Bogoriense 14 (2): 27-32
Dwiyani, R. (2012). Mikropropagasi tanaman anggrek Vanda tricolor Lindl. var suavis yang membawa gen Knotted 1-like Arabidopsis thaliana (KNAT1). Disertasi. Sekolah Pascasarjana
Universitas Gadjahmada, Indonesia (In Indonesian)
El-Siddig, M., El-Hussein, A., Siddig, M., Elballa, M., and Saker, M. (2009). Agrobacterium-Mediated Transformation and in Vitro Regeneration of Tomato
.(Lycopersicon Esculentum Mill.) Plants Cv. Castlerock. The Journal of Genetic Engineering and
Biotechnology 7(1): 1-6
Feldmann, K. (1992). T-DNA insertion mutagenesis in Arabidopis: seed infection transformation. In Koncz, C., Chua, N-H., and J. Schell, eds. Methods in Arabidopsis
Research.World Scientific,
Singapore. pp 274–289
Feldmann, K. A., and Marks, M. D. (1987). Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol. Gen. Genet. 208: 1–9
Fujita, K., Matsuoka, T., Suzuki, S., and Takayanagi, T. (2009). In Planta Transformation Technique for Grapevines (Vitis vinifera L) using Dormant Buds. J. Plant Biochem. and Biotech. 18 (2): 161-167
Hansen, G., and Wright, M. S. (1999). Recent advances in the transformation of plants. Trends Plant Sci. 4: 226– 231
Katavic, V., Haughn, G. W., Reed, D.,
Martin, M., and Kunst, L. (1994). In planta transformation of Arabidopsis thaliana. Mol. Gen. Genet. 245: 363– 370
Kim, S. I., Veena, and Gelvin, S. B. (2007). Genome-wide analysis of
Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J. 51: 779–791.
Kohler, F., Cardon, G., Pohlman, M., Gill, R., and Schieder, O. (1989). Enhancement of transformation rates in higher plants by low-dose
irradiation: are DNA repair systems involved in the incorporation of exogenous DNA into the plant genome? Plant Mol. Biol. 12: 189– 199.
Labra, M., Vannini, C., Grassi, F., Bracale, M., Balsemin, M., Basso, B., and Sala, F. (2004). Genomic stability in Arabidopsis thaliana transgenic plants obtained by floral dip. Theor. Appl. Genet.109 (7):1512-1518
Li, J., Tan, X., Zhu, F., and Guo, J. (2010). A Rapid and Simple Method for Brassica Napus Floral-Dip
Transformation and Selection of Transgenic Plantlets. International J. of Biol. 2 (1) :127-131
Liau, C. H., You, S. J., Prasad, V., Hsiao, H. H., Lu, J. C., Yang. N. S., and Chan, M. S. (2003). Agrobacterium tumefaciens-mediated transformation of An Oncidium Orchid. Plant Cell Reports 21 : 993-998
Mezzetti, B., Silvestroni, O., Costantini, E., Pandolfini, T., and Spena, A. (2005). Genetic transformation of table grape via organogenesis and field evaluation of defH9-iaaM transgenic plants. Acta Horticulturae 689: 463468
Park, S. Y., Vaghchhipawala, Z., Vasudevan, B., Lee, L. Y., Shen, Y., Singer, K., Waterworth, W. M., Zhang, Z. J., West, C. E., Mysore, K. S., and Gelvin, B. (2015). Agrobacterium T-DNA integration into the plant genome can occur without the activity of key non-homologous end-joining proteins. The Plant J. 81: 934–946
Paul, A., Bakshi, S., Sahoo, D. P., Kalita, M.C., and Sahoo, L. (2012).
Agrobacterium-mediated genetic
transformation of Pogostemon cablin (Blanco) Benth. Using leaf explants: bactericidal effect of leaf extracts and counteracting strategies. Appl.
Biochem. Biotechnol. 166 (8):1871-95.
Razzaq, A., Hafiz, I. A., Mahmood, I., and Hussain, A. (2011). Development of in planta transformation protocol for wheat. African J. Biotech. 10 (5): 740-750
Salomon, S., and Puchta, H. 1998. Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J. 17: 6086–6095.
Semiarti, E., Indrianto, A., Purwantoro, A., Isminingsih, S., Suseno, N., Ishikawa, N. T., Yoshioka, Y., Machida, Y., and Machida, C. (2007). Agrobacterium-mediated transformation of the wild orchid species Phalaenopsis amabilis. Plant Biotechnol..24 : 265-272.
Semiarti, E., Purwantoro, A., Mercuriani, I. S., Anggriasari, A. M., Jang, S., Suhandono, S., Machida, Y., and Machida, C. (2014). In planta transformation method for T-DNA transfer in orchids. In: AIP
Conference Proceedings 2014 Volume 1589 (Issue 1): 303-307
Sharafi, A., Sohi, H. H., Mirzaee, H., and Azadi, P. (2014). In vitro regeneration and Agrobacterium mediated genetic transformation of Artemisia aucheri Boiss. Physiol Mol Biol Plants 20(4):487–494
Stachel, S. E., Timmerman, B., and Zambryski, P. (1986). Generation of single-stranded T-DNA molecules during the initial stages of T-DNA transfer from Agrobacterium
tumefaciens to plant cells. Nature 322: 706–712.
Sugiharto, B., Sakakibara, Sumadi, and Sugiyama. (1997). Differential Expression of Two Genes for Sucrose-Phosphate Synthase in Sugarcane: Molecular Cloning of the cDNAs and Comparative Analysis of Gene Expression. J. Plant Cell Physiol. 38: 961-965.
Svabova, L., Smykal, P., Griga, M., and Ondreji, V. (2005). Agrobacterium-mediated transformation of Pisum
sativum. Biologia Plantarum 49 (3): 361-370
Tinland, B., Hohn, B., and Puchta, H. (1994). Agrobacterium tumefaciens transfers single-stranded transferred DNA (T-DNA) into the plant cell nucleus. Proc. Natl. Acad. Sci. USA 91: 8000– 8004.
Tzfira, T., Frankman, L. R., Vaidya, M., and Citovsky, V. (2003). Site-specific integration of Agrobacterium tumefaciens T-DNA via doublestranded intermediates. Plant Physiol. 133: 1011–1023.
Yusibov, V. M., Steck, T. R., Gupta, V., and Gelvin, S. B. (1994). Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc. Natl. Acad. Sci. USA, 91: 2994–2998.
Zhanga, Y., Zhanga, D., Zhong, Y., Changa, X., Hub M., and Chenga, C. (2017). A simple and efficient in planta transformation method for pommelo (Citrus maxima) using Agrobacterium tumefaciens. Scientia Horticulturae
214: 174–179
FACULTY OF AGRICULTURE, UDAYANA UNIVERSITY • 105
Discussion and feedback