APPLICATION OF Trichoderma spp. AND LIGNOHUMATE TO SUPPRESS A PATHOGEN OF CLUBROOT (Plasmodiophora brassicae WOR.) AND PROMOTE PLANT GROWTH OF CABBAGE
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • Vol. 6 No. 2 • April 2019 eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2019.v06.i02.p01
ARTICLE
APPLICATION OF Trichoderma spp. AND LIGNOHUMATE TO SUPPRESS A PATHOGEN OF CLUBROOT (Plasmodiophora brassicae WOR.) AND PROMOTE PLANT GROWTH OF CABBAGE
I Ketut Suada1*, Anak Agung Ngurah Gede Suwastika1, I Kadek Ngestika Pradnyana1, Nataliya Shchegolkova2, Rodion Poloskin3, Oleg Gladkov4, Olga Yakimenko5, and Aleksey Stepanov5
1Faculty of Agriculture, Udayana University, Bali, Indonesia 2Institute of Water Problems RAS, Moscow, Russia 3Lignohumate Company, St-Petersburg, Russia
-
4Ltd Scientific Productive Association Realization of Ecological Technologies, St-Petersburg, Russia 5Soil Science Faculty, Lomonosov Moscow State University, Moscow, Russia *Corresponding author: ketutsuada@unud.ac.id
ABSTRACT
The high economic value of cabbage crop leads farmers to make various efforts to suppress the pathogen of club root which is detrimental to plants. Efforts that need to be put forward must be environmentally safe. One way that is environmentally friendly is to control a pathogen biologically using antagonistic microbes. Therefore, the use of microbes such as Trichoderma which has been widely studied is important because it was able to suppress clubroot incidence and stimulate plant growth as well. Meanwhile, the need for plant nutrients to maximize plant growth requires an input of organic materials such as lignohumate which preserve soil nutrients, improve soil structure and increase plant resistance to biotic and abiotic stresses. The previous study on this scheme found an effective indigenous Trichoderma to suppress clubroot, therefore it is used in the current study. The objective of this study was to find out a combination treatment of Trichoderma and lignohumate which can suppress clubroot and increase plant growth. This experiment used a Randomized Block Design with 2 factors and 3 replications. Trichoderma concentration consisted of 3 levels, namely 0 spores (control), 1x106 spores. 2x106 spores, and 3x106 spores per plant which were suspended in 150 ml of water. The lignohumate treatments were 0.01, 0.02, 0.05, 0.1, 0.5, 1.0, and 2%. The results showed that lignohumate treatment was interact with Trichoderma population number on disease incidence, total clubroot, root dry weight, but not to canopy dry weight. The most suitable combination of treatments was the Trichoderma population of 3x106 spores (15 g) in combination to lignohumate of 0.5%. This combination resulted in the lowest disease incidence, the lowest total clubroot, root dry weight, and the highest canopy dry weight. The higher the lignohumate concentration up to 0.5%, the higher the number of microbes (fungi and bacteria) growth, howeverit decreased above the concentration of 0.5%.
Keywords: cabbage, clubroot, Trichoderma, lignohumate
INTRODUCTION
Cabbage production in Bali is spread throughout all nine regencies in Bali except
Jembrana, Klungkung, and Denpasar while the central production of cabbages is in Tabanan regency. Bali local cabbage
production from year to year since 2010 continues to decline. In 2010 cabbage production in Bali amounted to 47,077 tons, in 2011 to 42,926 tons, in 2012 amounted to 40,167 tons, and in 2013 it was only 35,781 tons (Bali Statistical Central Bureau, 2014). The decline in production is closely related to the attack of clubroot which is increasingly rampant in cabbage plants and even attacks other types. The attack of clubroot pathogens is always to be a problem to every cabbage production season and occurs in almost every cabbage planting area (personal
communication).
Cabbage clubroot is a disease of Brassicaceae family caused by the soil-borne fungi Plasmodiophora brassicae Wor. The disease first appears scattered in fields, but can infect the entire field, reducing the yield significantly and sometimes resulting in no yield at all. Symptoms appear as yellowing, wilting, stunting, and galls on the roots. It is transmitted by contaminated transplants, animals, surface water runoff, contaminated equipment, and irrigation water. The pathogen can survive in a field for years as resting spores without a host present and will infect the next susceptible crop.
Developing plants may not show any symptoms but as the plants get older they will start to show symptoms of chlorosis or yellowing, wilting during hot days, and exhibit stunted growth. Below ground, the
roots experience cell proliferation due to increased auxin or growth hormone production from the plant as well as the pathogen. The pathogen excrete methyl transferase (PbBSMT) which reduce production of Salicylic Acid (SA), an immunity plant defense, so that the clubroot formed in root. The clubroot might be resulted by interference of root phytohormone system combined with meristematic activity of the root (Ludwig-Muller, 2015; Malinowski, 2012).
The clubroot can grow big enough to restrict the xylem tissue inhibiting efficient water uptake by the plant. Galls appear like clubs or spindles on the roots. Eventually the roots will rot and the plant will die. Gall formation or distortion takes place on latent roots and gives the shape of a club or spindle. In the cabbage such attacks on the roots cause undeveloped heads or a failure to head at all, followed often by decline in vigor or by death. It is an important disease, affecting an estimated 10% of the total cultured area worldwide.
The high economic value of cabbage crops causes farmers to make various efforts to suppress the pathogen of clubroot. Efforts that need to be put forward must support sustainable agriculture and be environmentally safe. One way that is environmentally friendly is to control biologically using antagonistic microbes
(Punja and Utkhede, 2003; Tian et al., 2007). Therefore, the use of microbes such as Trichoderma which has been widely studied is important because it was able to suppress many pathogens such as P. brassicae (Xue-Xin Yu, 2015), Fusarium solani by 68.22% (Barari and Foroutan, 2013;), Rhizoctonia solani by 67.8-74.4% (Asad et al., 2014; Bastakoti et al., 2017). The advantage of Trichoderma application as biocontrol agent is stimulate plant growth as well (Sing et al., 2016; Patel et al., 2016). Mode of action of Trichoderma spp. is including to produce cell wall degrading enzymes as pectinase and chitinase, fast growing hypae to compete to other microbe, and able to produce antibiosis substance against pathogens (Munir et al., 2013; Sarma et al., 2014). Trichoderma atroviride produce cell wall degrading enzymes such as N-acetyl-β-d-glucosaminidase, 1,4-β-Chitobiosidase, endochitinase, and glucose oxidase which harms to pathogens (Brunner et al., 2005). Trichoderma spp. are free-living cosmopolitan species interacting with roots, soil, and foliar environments. Its mycelia is fast growing, therefore they compete strongly to other microorganisms. Furthermore, they degrade pectin and inhibit pectinases those are essential for plant-pathogenic fungi (Munir et al., 2013).
Meanwhile, the need to maximize plant growth requires nutrients which can be
derived from fertilizers that can be provided by organic materials such as humus or compost. Humic or compost affect plant physiology namely increase nutrient uptake, bind nutrients preventing leaching by run off water, increasing beneficial microbe, enhancing water preservation around root (Trevisan et al., 2010, Canellas et al., 2015). Humic substances based amendments as fertilizers have been increased in recent years and proven increase plant yield. Lignohumate (LH) is one of humic sources commercially is used widely in agriculture for many crops and various conditions. The advantage of LH is preserve nutrients of leaching, improve soil structure, and increase plant resistance to biotic and abiotic stresses. The previous year's study on this scheme have been found an effective indigenous Trichoderma to suppress clubroot and would be used in this study. The purpose of this study was to obtain the best combination treatment of Trichoderma and LH which able to suppress clubroot and also increase plant growth.
MATERIALS AND METHODS Treatments
The treatments tested in this experiment was population of Trichoderma as biocontrol agent in combination with doses of humic product Lignohumate (LH). The treatments were set according to a
Randomized Block Design with 3 (three) replications. The indicator plant was cabbage (Brassica oleracea L. var. capitata) which is susceptible to clubroot disease (field real experience).
Three days after transplanted, the lignohumete on each concentration were poured on around the root and one day after that the Trichoderma was poured for each population. The Trichoderma applied one time, however the lignohumate was applied for three times. The first was poured on three days after transplanted and the next two were on three and six weeks after transplanted by spraying to the leaves of growing cabbage.
Treatments for LH was the variation of concentration namely: 0.01, 0.02, 0.05, 0.1, 0.5, 1.0, and 2% (w/v) in water which were poured eventually after cabbage planted. “Lignohumate AM” is a commercial humic product with microelements, produced from lignin by an industrial process, containing about 80% of humic substances, 9% K, 3% S, 0.1-1.2 g kg-1 Mg, 0.1-2.0 g kg-1 Fe, 0.1-1.2 g kg-1 Cu and Co, 0.1-1.5 g kg-1 B, 0.05-1.15 g kg-1 Mo, 0.1-1.2 g kg-1 Zn (on dry matter basis).
The Trichoderma applied was T. asperellum (identification record ITS4/ITS5 CBS 433.97 ITS region, 99%) isolated from cabbage plantation and collected in Laboratoty of Biotechnology, Faculty of Agriculture, Udayana University. The
population treatment consisted of three levels, namely 0 inoculum (not spores nor mycelium, control), 1x106 spores, 2x106 spores, and 3x106 spores per plant (equal to 0, 5, 10, and 15 g of half-cooked rice with spores) which were suspended in 150 ml of water. The treatment without inoculum (control), plants were doused with 150 ml of water per plant according to the treatment combination.
Inoculum preparation
T. asperellum pure culture were inoculated into half-cooked rice aseptically. A half kilogram of the rice was put into plastic bag and shaking, then incubated for a week. When the blue colour appear on all around the rice, then the spores formed in it was measured by haemacytometer. In this research the amount of spores in one gram of the rice media was equals to 1 x 106 spores.
Seedling preparation
Cabbage seedling are prepared by means of seeds sowed on beds made of bamboo blades with a soil-humus mixture of 1:1 ratio. The beds are watered just enough everyday and after the seedling have grown and was 7 days old, the seedlings were ochered (soil fist at the root) and after 14 days were ready to be replanted to the field and arranged in a distance of 50 cm x 50 cm in a plot sized of 100 cm x 1200 cm.
Maintenance of plant
Plant maintenance was done to keep field capacity of humidity, removing weeds, and fertilization. All the plots (1 x 1.2 m2) were treated with basic NPK fertilization: Urea (46% N) and Ponska (15% N, 15% P2O5, and 15% K2O) with doses of Urea 2.4 g/plot + Ponska 2.4 g/plot which distributed two times. The application unorganic fertilizers were as follows: 1) one week after transplanting: 1.2 g urea/plot + 1.2 g
Ponska/plot, 2) five weeks after transplanting: 1.2 g urea/plot + 1.2 g
Phonska/plot.
Variables observed
During the experiment, leaf area and leaf chlorophyll content were measured at two time periods: 5 weeks and at 10 weeks after planting. After harvest the following variables were determined: disease incidence, total amount of galls on roots (clubroot), root dry weight, canopy (parts of the plant above the ground) dry weight, and microbe population including bacteria, fungi, and Trichoderma population. Chlorophyll content was measured using Chlorophyll Meter type SPAD-502 Plus (Konica Minolta) with its unit is SPAD.
Microbe plating
One gram of soil sample was diluted in stages to 10-1, 10-2, 10-3, 10-4, 10-5, 10-6,
and 10-7 in sterile water. One ml of each dilution was plated in different media on petri dish. Bacteria was plated in NA (Nutrient Agar Merck®: agar 15 g/l, beef extract 3 g/l, peptic digest of animal tissue 5 g/l, sodium chloride 8 g/l, final pH 6.0±0.2, 25oC; preparation was of 39 g powder in 1 litre of water) medium with 2% benzimidazole, while the fungus were isolated in PDA (Potato Dextrose Agar, Merck®: agar 15 g/l, dextrose 20 g/l, potato extract 4 g/l, pH 5.6±0.2, 25°C, preparation of 39 g in 1 litre of water) with 0.1% ciprofloxacyn-500). The colony appeared on medium were counted in colony counter apparatus, CFU/g soil.
Statistical analyses
The data were variant analysed according to the randomized complete design using the Microsoft Excel software. When the difference between the treatments was significant, this differences were compared by Duncan Multiple Range Test on level of 1 and 5%.
RESULTS AND DISCUSSION
Disease Incidence
The Lignohumate (LH) and Trichoderma interacted to variable of disease incidence. Without Trichoderma, the exhibited of disease was decrease by the increase of LH doses while the doses of LH
increase, however, in the increase of Trichoderma population was not cause the decrease of disease incidence until the population of 2x106 CFU (10 g of application) (Table 1). Then, the combination of treatments that gave the best effect was the Trichoderma treatment on population of 3x106 spores (15 g). In this population the least disease incidence (7.1%) gained in LH dose of 0.5%, while the control treatment was 88.1% disease incidence. This
is the best combination of LH concentration (0.5%) and population of Trichoderma (3x106 spores,15 g) which resulted to the lowest of disease incidence. When LH doses was increased from the dose of 0.5% in this the highest population, the disease incidence was increase significantly. This mean that, the doses more than 0.5% couldn’t support Trichoderma to eliminate the pathogen infection.
Table 1. Disease incidence of clubroot on cabbage under treatment of lignohumate and Trichoderma
|
Lignohumate |
Trichoderma sp. | |||||||
|
concentration (%) |
0 (0 g) |
1x106 |
(5 g) …… % … |
2x106 (10 g) |
3x106 (15 g) ………… | |||
|
0.00 |
8…8.…1 … |
a |
………8…0.…1 |
a |
74.0 |
a |
66.1 |
a |
|
0.01 |
62.5 |
a |
80.1 |
a |
74.0 |
a |
66.1 |
a |
|
0.02 |
72.2 |
a |
80.1 |
a |
66.1 |
a |
58.2 |
b |
|
0.05 |
72.2 |
a |
72.2 |
a |
74.0 |
a |
58.2 |
b |
|
0.10 |
72.2 |
a |
72.2 |
a |
74.0 |
a |
58.2 |
b |
|
0.50 |
72.2 |
a |
41.1 |
b |
66.1 |
a |
7.1 |
c |
|
1.00 |
49.1 |
b |
49.1 |
b |
66.1 |
a |
32.0 |
b |
|
2.00 |
55.2 |
b |
80.1 |
a |
66.1 |
a |
41.1 |
b |
Remarks: Means value with the same letter are not significantly different (p>0.05) based on Duncan’t Multiple Range Test.
The best combination of LH with Trichoderma occurred at 0.5% LH and 15 g of Trichoderma which resulted to the lowest incidence of disease infection. Novizan (2002) revealed that, Trichoderma spp. is the most widely studied about its ability to control fungi and plant-destroying bacteria. This fungus is a saprophytic fungus that lives on the ground and is easily mass produced with artificial media. Trichoderma spp. can
be hyperparasitic in some species of pathogenic fungi, its growth is very fast which makes possible to strong space competitor, and does not become a disease for higher plants. Therefore, when combined with LH, a synergism will occurred in suppressing clubroot. Rodion (2016) proved that LH has the ability of immunoregulators action which gives rise to the effect of plant resistance on various biotic and abiotic
stresses including P. brassicae. More over, according to Druzhinina et al. (2011); Shoresh et al. (2010); Kumar et al. (2017), Trichoderma spp. may form chemical compounds which promote plant defenses (MAMPs, Microbe Associated Molecular Patterns) such as peptaibols, xylanases, swollenins, and cerato-latanins.
Experimental field was heavily infected with clubroot, incidence of disease achieved 88% at the control treatment. When added separately, both tested products contributed to a decrease in the incidence of 2-9 times (88% to 49% in control, 66% to 7.1% in 15 g population of Trichoderma). Effect of Trichoderma alone decrease the disease was visible from 72.2% to 7.1% in 0.5% LH, whether LH alone at highest doses (1 and 2%) was able to decrease the pathogen infection. At combined application of Trichoderma with LH at rates higher then 0.02% the positive effect was much more pronounced, and in some cases the incidence of disease decreased up to 42-58%. The combination of treatments that gave the best effect was the Trichoderma treatment on population of 3x106 spores with LH-dose
0.5% which provided the least disease incidence (7.1%). However, at high doses of LH (1-2%) some growth of disease incidence was observed comparably to the one at lower rates (0.02-0.50%). This means that the doses more than 0.5% couldn’t support Trichoderma to eliminate the pathogen infection.
Total club root
At combined application of Lignohumate with the Trichoderma their interaction affected to club root formation (Table 2). The interaction which resulted to the lowest total club root (1 piece) was in the combined of 0.5% lignohumate and the Trichoderma population of 3x106 CFU (15 g) (Table 2), which was in accordance with the effect of the treatments on the disease incidence (Table 1). The lower percentage of pathogen attacks on plants resulted to lower number of club roots and dry weight as well. The lower dry weight (3.80 g, Table 4) on the best treatments because there was not occur hypertrophy and root cell hyperplasia which leading to cell remain healthy (Fig.1).
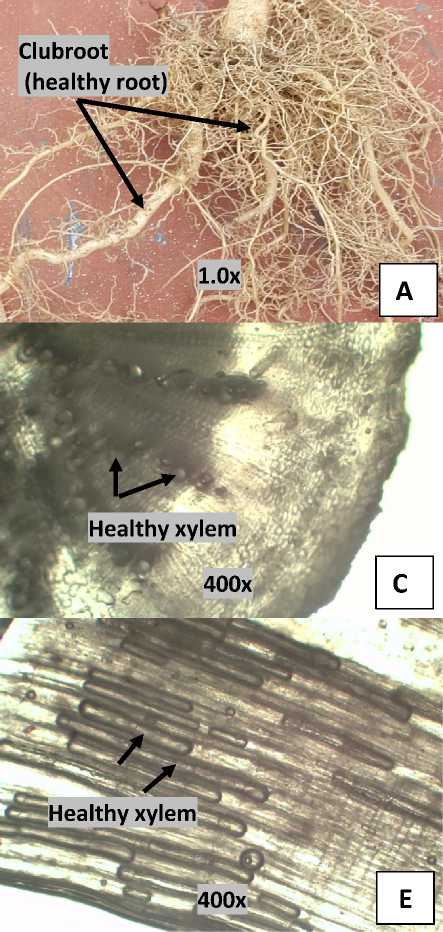
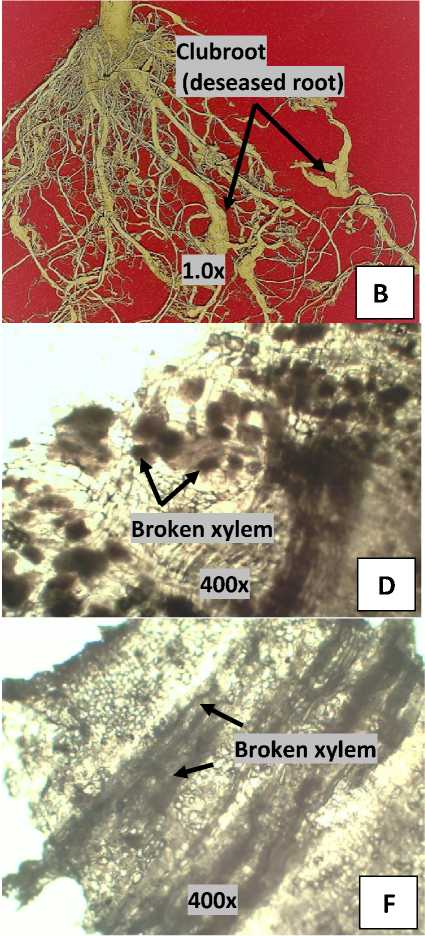
Fig. 1. Cabbage roots. Healthy roots (A), Infected roots (B), Cross section image of healthy root (C), Cross section image of infected root (D), Longitudinal section image of healthy root (E), Longitudinal section image of infected root (F). The arrow indicates xylem that has a change in the infected root (broken) and left is healthy image xylem.
Table 2. Total clubroot exhibited on the cabbage root on lignohumate and Trichoderma treatments
Lignohumate Trichoderma sp.
|
concentration (%) |
0 (0 g) |
1x106 |
(5 g) pieces per |
2x106 (10 g) plant ……………. |
3x106 (15 g) | |||
|
0,00 |
19,33 |
a |
……1.2.…,33 |
b |
10,33 |
b |
9,67 |
c |
|
0,01 |
12,00 |
b |
13,00 |
b |
10,33 |
b |
10,00 |
b |
|
0,02 |
10,67 |
b |
13,67 |
b |
8,67 |
c |
7,33 |
c |
|
0,05 |
10,67 |
b |
11,33 |
b |
8,67 |
c |
8,00 |
c |
|
0,10 |
11,33 |
b |
12,00 |
b |
10,33 |
b |
8,33 |
c |
|
0,50 |
11,33 |
b |
4,33 |
de |
8,67 |
c |
1,00 |
f |
|
1,00 |
6,00 |
cd |
7,33 |
cd |
9,00 |
c |
5,67 |
de |
|
2,00 |
8,33 |
cd |
14,33 |
b |
9,33 |
c |
6,00 |
cd |
Remarks: Means value with the same letter are not significantly different (p>0.05) based on Duncan’t Multiple Range Test.
Besides of disease incidence, both LH affected also the root galls formation, reducing amount of clubs on cabbage roots by 3 times on treatment without Trichoderma (19.33 pieces clubs in 0% LH compared to 6 pieces in 0.5% LH) and decreased 10 times clubs on Trichoderma for 3x106 spores (9,67 pieces clubs in 0% LH compared to 1 pieces in 0.5% LH) (Table 2). LH enhanced the effect of Trichoderma and at the most effective treatment (Trichoderma population
of 3x106 CFU with 0.5% lignohumate), the minimum amount of galls was observed 1 against 19 pieces in control. This is in accordance with the effect of the treatments on the disease incidence, as well as “opposite effect” of high concentrations of LH (Table 1). This figures indicated that the LH treatment to the soil has significant effect to the immune system plant (immunomodulator effect) as stated by Poloskin (2012).
Table 3. Total of root dry weight of cabbage under lignohumate and Trichoderma treatments
Lignohumate Trichoderma sp.
concentration 0 (0 g) 1x106 (5 g) 2x106 (10 g) 3x106 (15 g)
(%)
……………….…....…….……. g ……….………………………………..
|
0,00 |
17,11 |
gh |
25,54 |
a |
14,82 |
abcdefgh |
15,55 |
abcdefg |
|
0,01 |
23,93 |
abc |
23,43 |
abcd |
16,90 |
abcdefg |
13,64 |
bcdefgh |
|
0,02 |
12,58 |
efgh |
15,30 |
abcdefg |
13,58 |
bcdefgh |
20,45 |
abcdefg |
|
0,05 |
15,73 |
abcdefgh |
22,07 |
abcdefg |
22,51 |
abcde |
20,29 |
abcdefg |
|
0,10 |
13,52 |
bcdefgh |
24,12 |
ab |
15,54 |
abcdefgh |
19,38 |
abcdefg |
|
0,50 |
20,58 |
abcdefg |
11,53 |
fgh |
12,96 |
defgh |
3,80 |
i |
|
1,00 |
25,54 |
a |
13,91 |
bcdefgh |
13,36 |
cdefgh |
11,73 |
fgh |
|
2,00 |
14,97 |
abcdefgh |
22,17 |
abcdef |
12,78 |
defgh |
11,98 |
efgh |
Remarks: Means value with the same letter are not significantly different (p>0.05) based on Duncan’t Multiple Range Test.
Root dry weight
High root dry weight was a result of the formation of high root cell volumes that occur due to root cell division which influenced by of the pathogen P. brassicae. When the pathogen interacts with its host, auxin will soon formed in plant cells, resulting in uncontrolled root cell division and tumor formation. High root dry weight indicates that the treatment is ineffective as in control. So the lower the dry weight of the root (to a certain extent), the more effective the treatment. Effective treatment of pressing clubroot according to low root dry weight and the most effective treatment was 0.5%
LH dose combination with Trichoderma concentration of 3x106 (15 g) which produced 3.8 g of root dry weight.
Canopy dry weight, leaf area, and chlorophyll content
The highest canopy dry weight also occurs at 0.5% LH concentration in the entire Trichoderma population (Fig. 2) and also the largest leaf area occurred at the lignohumate dose (Fig. 3). The highest chlorophyll amount achieved was 7400.77 SPAD at the same lignohumate dose which was higher than that at 1.0% (Figure 4) which the amount of Trichoderma 3x106 CFU (15 g).
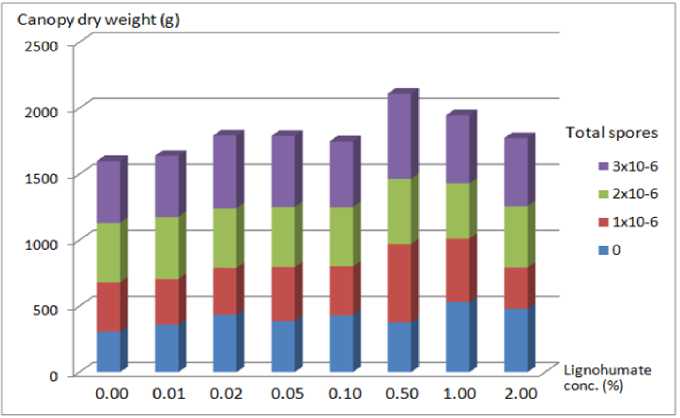
Fig. 2. Canopy dry weight under LH concentration and total Trichoderma spores.
The highest dry weight canopy occurred at 0.5% LH concentration and the lowest was in the control treatment (Fig. 2). This showed that LH has an active role in increasing soil fertility, increasing nutrient
uptake, and also increasing the amount of chlorophyll, all of which will produce high photosynthetic products that were expressed in the high dry weight of plants.
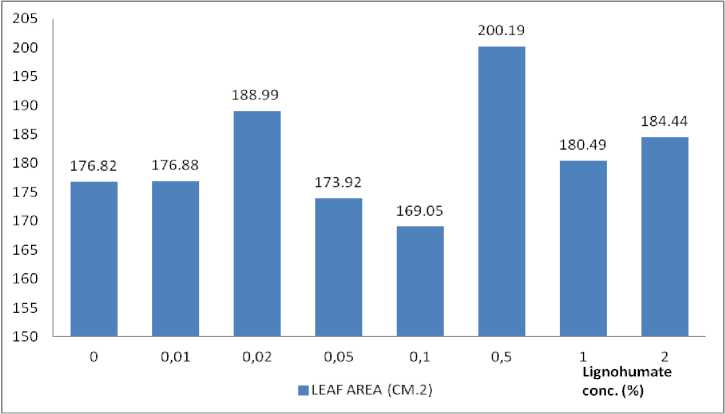
Fig. 3. Leaf area under LH concentration ignoring total Trichoderma spores.
Based on Fig. 3, it was seen that LH also spurred the formation of leaf area which is the highest (200.19 cm2) at the concentration of 0.5% LH.
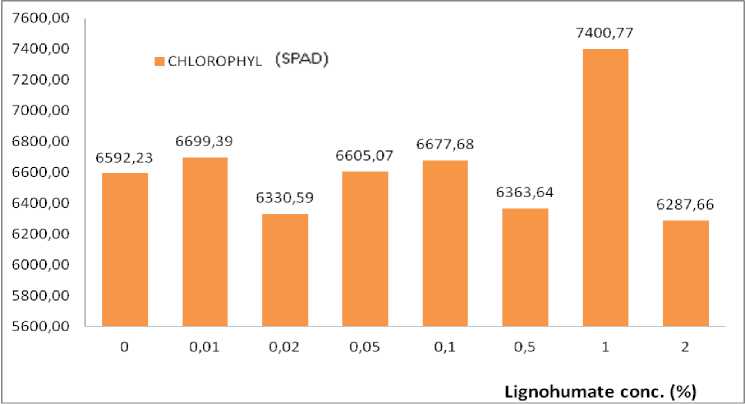
Fig. 4. Chlorophyll content under LH concentration ignoring total Trichoderma spores.
Microbial population
Humic substances are showed to increase soil biological activity, and this is considered to be one of the probable mechanisms of their positive effect on plant growth and development. This is true for humic-based product LH as well. Table 4
shows the effect of different concentrations of LH on microbial population in soil. It mostly stimulated the bacterial population, and the concentration of 0.5% provided the highest increment of bacterial and fungi population.
Table 4. Total microbe exhibited in the cabbage media culture under Trichoderma and LH
treatment
|
Lignohumate concentration (%) |
Microbe population | ||
|
Bacteria (spp.) (1x106 CFU) |
Fungi (spp.) (1x106CFU) |
Trichoderma spp. (1x106 CFU) | |
|
0,00 |
10±0,67 d |
13±0,33 b |
1,5±0,40 c |
|
0,01 |
33±0,33 b |
10±0,56 b |
3,0±0,20 b |
|
0,02 |
19±0,67 c |
10±0,43 b |
3,1±0,30 b |
|
0,05 |
28±0,37 b |
12±0,52 b |
4,0±0,24 b |
|
0,10 |
27±0,33 b |
11±0,23 b |
3,3±0,43 b |
|
0,50 |
39±0,67 a |
19±0,34 a |
5,7±0,82 a |
|
1,00 |
20±0,63 c |
11±0,22 b |
3,3±0,43 b |
|
2,00 |
27±0,33 b |
11±0,33 b |
3,3±0,82 b |
Remarks: Means value with the same letter are not significantly different (p>0.05) based on Duncan’t Multiple Range Test.
The LH on 0.05% concentration application was able to promote the dry weight of seedling of some cabbage family plant (Lactuca sativa, Eruca sativa, Coriandrum sativum, Brassica oleracea var. capitata) as twice of non treated plant (Suada et al., 2017). Lignosulfonate-humate-a and lignosulfonate-humate-b elicited hormonelike activity and leonardite displayed giberellin properties and subsequently increase the growth (Ertani et al., 2011).
LH can stimulate microbial growth of up to 0.5% concentration. The higher the LH concentration, the higher the number of microbes (fungi and bacteria) population, however, after a concentration of 0.5% LH, the microbial population decreases. According to Yakov (2010) that organic matter in the soil will invite microbes to grow because microbes get food through the
decomposition of these organic materials. In fertile soils, which contain high organic ingredients there are 100 million microbes of various types per gram of soil. In this study, increasing lignohumate will increase the number of microbes to a concentration of 0.5%, however, starting concentrations of greater than 0.5% the microbial population decreases. This contradicts to the statement that in more fertile soils more microbes inhabit the soil due to some disadvantageous happened in this condition. The LH concentration was not supported the growth of microbes.
The effect of Trichoderma was exhibited by many researcher that the fungi has some advantages through directly and undirectly in influencing pathogen and its host. Direct mechanisms is mycoparasitism, the Trichoderma’s mycelia titling other fungi mycelia and such its plasma till suffering and
dried. In this case the Plasmodiphora brassicae was not have a mycelia so this mechanism can’t be applied in this problems. Indirectly ways is competing for space and also nutrients, promoting plant defensive ability, antibiosis and moreover promoting plant growth (Benitez et al., 2004; Bastakoti et al., 2017 ). The biocontrol processes occurred may coordinately and its effectiveness is defend on the strain of Trichoderma, the antagonized fungus in the rhizoplane, the crop plant, and plant cirscumstance. The environmental factors which influenced to the control system is nutrient availability, iron concentration, pH, and temperature. Moreover, activity of each mechanisms will promote the production of some antibiosis compound such as plant growth factor, siderophore, antibiotic, hydrolytic enzymes, gliotixin (produced by T. virens) and also carbon and nitrogen elements. The enzymes produced are able to hydrolyze chitin, protein, cellulose, and hemicellulose of cells (Canellas et al., 2015).
LH was able to increase the synthesis of chlorophyll in cabbage plants. The highest chlorophyll formation (7400.77 SPAD in Fig. 4) was achieved at 1% LH dose in this study. The increased due to LH also occurs when LH was sprayed onto leaves, it was able to increase photosynthesis rates because higher chlorophyll content achieved. The same phenomenon is reported for various
plants such as pepper (Karakurt et al., 2009) and chrysanthemum (Fan et al., 2014). More over, according to Ertani et al. (2011) and Poloskin et al. (2012), LH has been shown to increase chlorophyll production. High chlorophyll has more implications for more photosynthesis which results in the formation of more carbohydrate products which means the dry weight of plants will be greater than without LH.
Trichoderma species is capable of producing 100 antibiotic metabolites including polyketides, pyrones, terpenes, which are derivative metabolites from amino acids and polypeptides (Sivasithamparam and Ghisalberti, 1998). Trichoderma as a biocontrol agent has been effective against pathogens such as Pythium aphanidermatium, Gaeumannomyces graminis var. tritici, Fusarium oxysporum, Rhizoctonia solani, Fusarium culmorum, Sclerotium rolfsii, Phytophthora cactorum, Botrytis cinerea and several Alternaria species (Dolatabadi et al., 2011; Bastakoti et al., 2017). Role of Trichoderma not only suppresses the growth of pathogens, but there are other influences that are beneficial for plants, namely increasing plant defenses, stimulating colonization by the rhizosphere microbes and stimulating plant root growth (Vinale et al., 2008).
CONCLUSIONS
The combination of the Trichoderma treatment of population of 3x106 spores (15 g with the treatment of LH of 0.5% resulted in the lowest root fresh weight, the lowest of root dry weight, the highest canopy fresh weight, the highest canopy dry weight, the lowest number of clubroot, and the lowest percentage of disease attack, indicated that this combination was the most suitable treatment in suppressing clubroot attack and promote plant growth of cabbage. The higher the lignohumate concentration up to 0.5%, the higher the number of microbes growth.
ACKNOWLEDGEMENTS
We express my great thank to Dean of Faculty of Agriculture and Head of Institute for Research and Community Service-Udayana University for the facilities prepared which promote this research activities well done. Thank you also to the Russia-ASEAN Project for moral and financial support so that this research moves forward and was completed on time. This research certainly will not work without the support of RET Ltd (Mr. Rodion Poloskin and Mr. Gladkov), namely in the form of providing lignohumate samples and application recommendations.
REFERENCES
Asad, S.A., Ali, N., Hameed, A., Khan, S.A., Ahmad, R., Bilal, M., Shahzad, M., & Tabassum A. (2014). Biocontrol efficacy of different isolates of
Trichoderma against soil borne
pathogen Rhizoctonia solani. Pol. J. Microbiol., 63(1), 95-103.
Barari, H. & Foroutan, A. (2013). Biocontrol of soybean charcoal root rot disease by using Trichoderma spp. Agronomic research in Moldavia, 4555. Available from:
https://www.researchgate.net/publicat ion/322162518, Trichoderma species as Biocontrol Agent against Soil Borne Fungal Pathogens.
[03/02/2019].
Bastakoti, S., Belbase, S., Manandhar, S., & Arjyal, C. (2017). Trichoderma species as biocontrol agent against soil borne fungal pathogens. Nepal Agricultural Research Council. DOI: 10.3126/njb.v5i1.18492.1.16.
Brunner, K., Zeilinger, S.R., Ciliento, Sheridian, L. W., Lorito, M., Kubicek, C.P., & Mach, R.L. (2005). Improvement of the fungal biocontrol agent Trichoderma atroviride to enhance both antagonism and induction of plant systemic disease resistance. Applied and
Environmental Microbiology, 71(7), 3959-3965.
DOI: 10.1128/AEM.71.7.3959-3965.2005. PMCID: PMC1168994. PMID: 16000810.
Canellas L.P., Olivares, F.L., Aguiar, N.O., Jones, D.L., Nebbioso, A., Mazzei, P. & Piccolo, A. (2015). Humic and fulvic acids as biostimulants in horticulture. Scientia Horticulturae, 5(196), 15-27.
Dolatabadi, K. H., Goltapeh, E. M., Varma, A. & Rohani, N. (2011). In vitro evaluation of arbuscular mycorrhizal-like fungi and Trichoderma species against soil borne pathogen. Journal
of Agricultural Technology, 7(1), 7384.
Druzhinina, I. S., Seidl-Seiboth, V., Herrera-Estrella, A., Horwitz, B.A.,
Mukherjee, P.K., Zeilinger,S.,
Grigoriev, I. V., & Kubicek, C. P. (2011). Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol., 9, 749-759.
Ertani, A., Francioso, O.V., Tugnoli, Righi, Nardi, V.S. (2011). Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem., 59, 11940-11948.
Fan, H., Wang, X., Sun, X., Li, Y., Sun, X., Zheng, C. (2014). Effect of humic acid derived from sediments on growth photosynthesis and
chloroplast ultrastructure in
Chrysanthemum. Scienta Hort.,
5(177), 118-123.
Karakurt, Y., Unlu, H. Padem, H. (2009). The influence of foliar and soil fertilization of humic acid on yield and quality of pepper. Acta Agric. Scand. Sect B, 5(59), 233-237.
Kumar, G., Maharshi, A. Patel, J., Mukherjee, A., Singh, H. B., &
Sarma. B. K. (2017). Trichoderma: A potential fungal antagonist to control plant diseases. Annual Technical Issue, 21. ISSN 0971-975X.
Ludwig-Muller, J., Julke, S., Geib, K., Richter, F., Mithofer, A., Sola, I., Rusak, G., Keenan, S., & Bulman, S. (2015). A novel methyltransferase from the intracellular pathogen Plasmodiophora brassicae
participates in methylation of salicylic acid. Mol. Plant Pathol., 16(4), 349364.
Malinowski, R., Smith, J.A., Fleming, A.J., Scholes, J.D., & Rolfe, S.A. (2012). Gall formation in clubroot infected Arabidopsis results from an increase in existing meristematic activities of the host but is not essential for the
completion of the pathogen life cycle. Plant Jour., 71, 226-238.
Novizan. (2002). Membuat dan
Memanfaatkan Pestisida Ramah Lingkungan. Jakarta. PT AgroMedia Pustaka.
Patel, J. S., Sarma, B. K., Singh, H. B., Upadhyay, R. S., Kharwar, R.N., & Ahmed, M. (2016). Pseudomonas
fluorescens and Trichoderma asperellum enhance expression of á subunits of the pea heterotrimeric G protein during Erysiphe pisi infection. Front. Plant Sci., 6,1206.
Poloskin R.B., Gladkov, O.A., Osipova, O.A.
& Yakimenko, O.S. (2012). Comparable evaluation of biological activity of new liquid and dry modifications of the humic product Lignohumate In Xu Jianming, Wu Jianjun, & He Yan (Eds.), Functions of natural organic matter in changing environments, Springer-Verlag
GmbH China. Proceeding of IHSS, 16, 619-621.
Punja, Z.K. & Utkhede, R.S. (2003). Using fungi and yeasts to manage vegetable crop diseases. Trends Biotechnol., 21, 400-407.
Sarma, B.K., Yadav, S.K., Patel, J.S., & Singh, H.B. (2014). Molecular mechanisms of interactions of Trichoderma with other fungal species. Open Mycol J., 8, 140-147.
Shoresh, M., Harman, G. E., & Mastouri, F. (2010). Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol, 48, 21-43.
Sivasithamparam, K., & Ghisalberti, E. L. (1998). Secondary metabolism in Trichoderma and Gliocladium. In G.E. Harman & C.P. Kubicek (Eds.), Trichoderma and Gliocladium. London: Taylor and Francis. pp.139192. [Google Scholar].
Suada, I. K., Rai, N., Budiasa, I.W., Santosa, N.G., Sunarta, N., Adnyana, G.M., Schegolkova, N.M., Poloskin, R.B.,
Gladkov, O.A., Yakimenko, O.S.
-
(2017) . Effect of lignohumate on yield and quality of rice in a paddy field in Bali, Indonesia. Water: Chemistry and Ecology, 5(107), 311.
Tian, B., Yang, J. & Zhang, K.Q. (2007).
Bacteria used in the biological control of plant-parasitic nematodes
populations, mechanisms of action, and future prospects. FEMS
Microbiol Ecol., 61, 197-213.
Trevisan, S., Francioso, O., Quaggiotti, S., Nardi, S. (2010). Humic substances biological activity at the plant-soil interface: from environmental aspects to molecular factors. Plant Signaling
& Behav., 5(6), 635-643.
Vinale, F., Sivasithamparam, K., Ghisalberti,
L.E., Marra, R., Woo, L.S. & Lorito,
M. (2008). Trichoderma-plant-
pathogen interactions. Soil Biol Biochem., 40, 1-10.
Xue-Xin Yu, Yong-Tian Zhao, Juan Cheng,
& Wei Wang. (2015). Biocontrol
effect of Trichoderma harzianum T4 on brassica clubroot and analysis of rhizosphere microbial communities based on T-RFLP. Biocontrol Science and Technology, 25(12), 1493-1505.
Yakov, K. (2010). Priming effects:
Interactions between living and dead organic matter. Soil Biology & Biochemistry, 42, 1363-1371.
94 • FACULTY OF AGRICULTURE, UDAYANA UNIVERSITY
Discussion and feedback