THE DEVELOPMENT OF OVARIAN FOLLICLE CELLS AND CORPUS LUTEUM OF MICE (Mus musculus) SWISS WEBSTER GIVEN Leucaena leucocephala LEAF EXTRACT
on
THE DEVELOPMENT OF OVARIAN FOLLICLE CELLS AND CORPUS LUTEUM OF MICE (Mus musculus) SWISS WEBSTER GIVEN Leucaena leucocephala LEAF EXTRACT
Ngurah Intan Wiratmini*, Ni Wayan Sudatri, and Iriani Setyawati Biology Departement, Faculty of Mathematic and Natural Science Of Udayana University
*Corresponding author : wiratminiintan@yahoo.com
ABSTRACT
This research was conducted to determine the effect of Leucaena leucocephala leaf extract on the development of ovarian follicle cells of mice (Mus musculus). Twenty four female mice, 10 weeks old, were divided into 4 groups. The control group (P0) were given 0.9% NaCl and the treatment group P1, P2 and P3 were given 0.5, 1 and 1.5 g/ kg bw of Leucaena leaf extract, respectively. The treatments were administered daily for 15 days by gavage. After the treatment ended, all mice were dissected to collect the ovaries. Histological preparation of the ovaries used paraffin method and Hematoxylin-Eosin staining. Observations were made on the number of primary, secondary, and tertiary oocytes, and also De Graaf follicles and corpus luteum. Data were statistically analyzed using One Way ANOVA method. The results showed there were no significantly differences (P>0.05) on the number of primary, secondary, and tertiary oocytes, as well as De Graaf follicles and corpus luteum among the control and treatment groups. The mimosine content of Leucaena leaf extract was 0.87% (less than 1%) so it could not inhibit the secretion of Follicle Stimulating hormone by the pituitary gland. The doses of Leucaena leaf extract used in this research did not significantly affect the follicles development of mice ovaries. In this study, the mimosine content of Leucaena leaf extract decreased 91.8%.
Keywords: Leucaena leucocephala, mice, ovary.
INTRODUCTION
Leucaena leucocephala, a Leguminoceae forage, was commonly used by farmers to feed both of their ruminant and non-ruminant cattle. One best advantage of this plant as a cattle forage is its high crude protein content (CP) 25-35% (Monoj et al., 2007). However, Leucaena utilization is limited because it contains an antinutrition substance called mimosine (Aung et al., 2006). Leucaena medicinal ingredients are known to cure stomach ache and as an anthelmintic (Saroso and Soenardi, 1995), relieve kidney inflammation, dysentery, insomnia, as well as cover the wound and laxative menstruation (Wijayakusuma, 2005). Giving 0.5 and 1 g/ kg bw of Leucaena seed extract orally to rats could
lower blood sugar levels (Hardani et al., 1991). Because of its mimosine content, it is necessary to analyze how Leucaena leaf could affect physiology of the body.
Mimosine, an alkaloid, are antimitotic thus affecting the synthesis and function of proteins in regulating the translation of mRNA and furthermore can inhibit DNA replication (Wang et al., 2000). Other studies have reported that mimosine could inhibit the cleavage of mouse embryos stage from 2 to 4 cells (Quhibi et al., 1994). This research was conducted in order to know whether Leucaena leaf extract can inhibit the growth of cells in the ovarian follicles of mice and to know the level of mimosine content of Leucaena leaf extract in thus study.
Table 1. Mimosine Content of the Leucaena leucocephala Leaf
|
Mimosine (%) |
Leucaena fresh leaf |
Leucaena leaf extract |
|
10.60 |
0.87 |
MATERIALS AND METHODS
This study used a Completely Randomized Design (CRD), consisted of four female mice groups, age 10 weeks (6 mice as replication in each group). One group as control (P0) was given 0.9% NaCl, and the treatment groups (P1, P2, P3) were given 0.5, 1 and 1.5 g/ kg bw of Leucaena leaf extract, respectively. Leucaena leaf extract was dissolved in 0.9% NaCl and given orally 0.2 ml/ day for 15 days. One day after the treatment ended, the animals were sacrificed and dissected for histological preparation of the ovaries (paraffin method, Hematoxylin and Eosin staining).
The extraction of Leucaena leaves followed the method of Harborne (1987), and further analysis of mimosine content followed the procedure of PAU Laboratory, Bogor Agriculture Institute, Indonesia. Parameters measured were the number of primary, secondary, tertiary and De Graaf follicles, as well as corpus luteum. Data
were analyzed using One Way Anova if the data distributed normally and had homogeneous variance, then followed by Duncan Multiple Range Test (DMRT) test if there was a significant difference (P <0.05).
RESULT AND DISCUSSION
The mimosine content of Leucaena leaf extract decreased by 91.8% compared with the fresh leaf of Leucaena (Table 1). This could happen because the usage of ethanol during the maceration process of extraction could dissolve the mimosine. According to Adenula et al. (2005), mimosine contains a polar fragment in its chemical structure; therefore a long maceration process using ethanol could dissolve the mimosine. Wiratmini et al. (2014) also reported that soaking in water for 12 hours could reduce the mimosine level of Leucaena leaves up to 73%.
Results of this study differed from those reported by Priatini (1999) that the provision of 10-40% Leucaena leaf of total feed in female mice decreased the uterus weight and led to necrosis of the ovarian membrane thus decreased follicle production (Figure 1). Other studies have also reported that 2-4% Leucaena seed extract in feed decreased sperm quality (Priastini and Rumiati, 2001).
Table 2. Average Number of Primary, Secondary and tertiary Oocytes, de graaf and Corpus Luteum of Mice Given Leucaena leucocephala Leaf Extract
|
Treatments |
Primary Oocyte |
Secondary Oocyte |
Tertiary Oocyte |
De Graaf Follicle |
Corpus Luteum |
|
P0 |
27,00 ± 2,00a |
7,50 ± 1,05a |
2,33 ± 0,82a |
1,17 ± 0,75a |
2,83 ± 0,98a |
|
P1 |
27,67 ± 2,16 a |
7,67 ± 1,03 a |
2,33 ± 0,52 a |
1,67 ± 1,03 a |
2,83 ± 0,75 a |
|
P2 |
24,83 ± 2,93 a |
6,17 ± 0,40 a |
1,83 ± 0,41 a |
2,17 ± 0,98 a |
2,50 ± 0,54 a |
|
P3 |
24,83 ± 2,56 a |
6,83 ± 1,17 a |
1,83 ± 0,98 a |
1,66 ± 0,52 a |
2,17 ± 0,75 a |
Different letters within a column indicate a significant differences (P<0.05)
INTERNATIONAL JOURNAL OF BIOSCIENCE AND BIOTECHOLOGY • VOL. IIi NO. 1 • SEPTEMBER 2015 ISSN: 9 772302 257 000
ISSN ONLINE: 9 772303 337 008
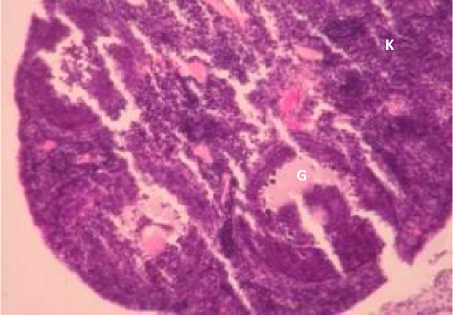
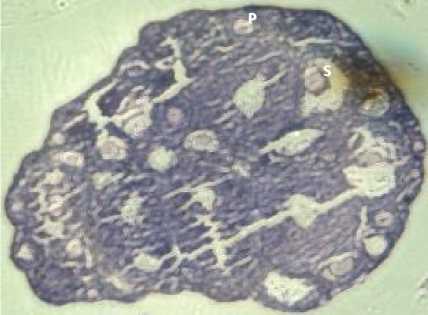
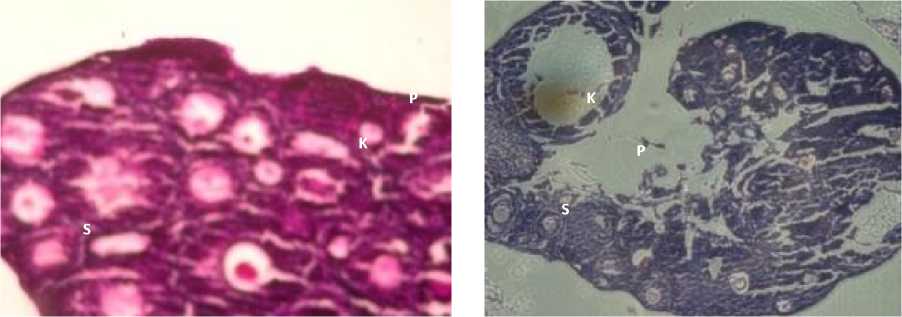
Fig. 1. Ovarian Transverse Tection in K (A), P1 (B), P2 (C) and P3 (D), P: Primary Oocyte, S: Secondary Oocyte, T: Tertiary Oosit, D: De Graaf, K: Corpus Luteum,
The reproduction quality deterioration occured because the mimosine content in the Leucaena leaves and seeds could inhibit FSH secretion.
According to Chancay and Poosaran (2009), administration of Leucaena leaf meal to monogastric animals should not be more than 10% of the total feed. If Leucaena fresh leaves contain 10.64% mimosine, the mimosine level of feed containing 10% Leucaena leaf meal was 1.06%. In line with that reported by Fayemi (2011), that rabbits are not tolerant to diet containing more than 1% of mimosine. Rabbits are monogastric animals as well as mice that used in this study.
CONCLUSIONS
Leucaena leaf extract at doses 0.5, 1 and 1.5 g/ kg bw did not inhibit the
development of follicle cells in mice ovaries. There was a decrease of 91.8% mimosine content of Leucaena leaf extract in this study.
REFERENCES
Adenula, I.O., Akanbi, A.I., Idowu, S.O. 2005. Comparative Nematocidae activity of Chromatographic Fructions of Leucanea leucocephala Seed Against Gastrointestinal Sheep Nematodes. Pharmaceutical Biology. Vol.43 Issue 7. 599-604
Aung, A., T. Ngwe, U. Ter Meulen, F. Gessler, & H. Bohnel. 2006. Control of leucaena toxicosis in Myanmar sheep using IBT gottinger-bioreactor grown mimosine degrading ruminal Klebsiella spp. Conference on International Agriculural Research for Development, Tropentag.
Chanchay, N. and Poosaran, N. 2009. The reduction of mimosine and tannin contents in leaves of Leucaena leucocephala. Asian Journal of Food and Agro-Industry. Special Issue, p. 137-144.
Fayemi, P.O., Onwuka, C.F.I., Isah, O.A., Jegede, A.V., Arigbede, O.M and Muchenje, V. 2011. Effects of mimosine and tannin toxicity on rabbits fed processed Leucaena leucocephala (Lam) De Wit. Leaves. African J. Of Agri. Research. 6 (17) : 4081-4085.
Harbone, J.B. 1987. Metode Fitokimia: Penuntun Cara Modern Menganalisa Tumbuhan. ITB Bandung. Alih Bahasa Padmiwinata, K & I. Soediro.
Hardani, N., Soegiarso, N.C., Ranti, A.S. 1991. Pengujian Efek Ekstrak Biji Leucaena leucocephala (Lam) De Wit terhadap Kadar Glukosa darah Tikus. Sekolah Farmasi ITB http://bahan-alam.fa.itb.ac.id. Bandung
Monoj, K., Ghosh & Bandyopadhyay, S. 2007. Mimosine Toxicity-A Problem of Leucaena Feeding in Ruminants. Asian Journal of Animal and Veterinary Advances 2 (2): 63-73.
Ouhibi, N., Fulka,J., Kanka, J and Moor, R.M. 1994. A Reversible block at the G1/S border during cell cycle progression of mouse embryos. The International Journal of
Developmental Biology 38(4).
Priastini, R. 1999. Efek Toksik Daun Lamtoro (Leucaena leucocephala) Terhadap Organ Reproduksi Mencit Betina (Mus musculus). Meditek. 7 (20): 38-46.
Priastini, R., dan Rumiati, F. 2001. Efek Spermatisida Ekstrak Biji Lamtoro Gung terhadap Kualitas sperma Manusia. In Vitro. Meditek. 9 (26): 26-34
Saroso, B., Soenardi. 1995. Lamtoro Sebagai Tanaman Obat Tradisional di Ngambre, Ngawi. Prosiding Seminar Etnobotani: 206
Wang, G., R. Miskimins, & W. K. Miskimins. 2000. Mimosine arrests cells in G1 by enhancing the levels of p27 (Kip 1). Exp. Cell Res. 254: 64-71.
Wijayakusuma, H. 2005. Sehat dengan Lamtoro. Available at:
http://www.suarakarya.online.com/n ews.html?id. Opened. 9.09.2013
Wiratmini, N.I., Oka, I.L., Putra, S., and Mahardika, I.G. 2014. The Effect of Detoxificated Leucaena
leucocephala Leaf Meal to Prenatal Development of Wistar Rat Fetus. Int. J. Pure App. Biosci. 2 (6): 223227
ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM • 31
Discussion and feedback