MULTIPLEX PCR FOR DETECTION OF CAPSULAR POLYSACCHARIDES TYPES OF Streptococcus pneumoniae CLINICAL ISOLATES IN BALI
on
MULTIPLEX PCR FOR DETECTION OF CAPSULAR POLYSACCHARIDES TYPES OF Streptococcus pneumoniae CLINICAL ISOLATES IN BALI
Ni Nengah Dwi Fatmawati1,2,3*, Ni Made Adi Tarini1,2, and I Putu Bayu Mayura1 1Department of Clinical Microbiology, Medical School, Faculty of Medicine, Udayana University 2Clinical Microbiology Laboratory, Sanglah General Hospital 3Molecular Biology Laboratory, Faculty of Medicine, Udayana University *Corresponding author : nnd.fatmawati@unud.ac.id
ABSTRACT
Streptococcus pneumoniae is causative agent of non-invasive and Invasive Pneumococcal Diseases (IPD). One of the major virulence factors is capsular polysaccharides (CPS). The CPS is known as the pneumococcal vaccine component. Several types of S. pneumoniae CPS are dominant in Indonesia such as types 6, 23, 15, 33 and 12 in West Nusa Tenggara, type 7F in Jakarta, and types 6A/B dan 15B/C in Central Java. No data is reported from Bali related to S.pneumoniae CPS typing. Therefore, the aim of this study was to determine CPS types of S. pneumoniae isolates in Clinical Microbiology Laboratory, Sanglah General Hospital, Denpasar, Bali by using Multiplex PCR. Twenty-one isolates that were isolated from blood (11/52.4%), sputum (5/23.8%), and other clinical specimens (5/23.8%) were included in this study. Identification of S. pneumoniae was based on optochin test and presence of pneumolysin gene (ply). Uniplex PCR was conducted to determine capsular type of each isolates, and then continued with Multiplex PCR 1 and 2, which used in-house positive controls. All isolates were positive for the presence of ply, confirming the isolates were S. pneumoniae. Moreover, this study showed that type 19F was the predominant type (7 isolates (66.7%)); 2 isolates (9.5%) were positive for each type 23F and also for type 6A/B; and, there was only 1 isolate (4.8%) for each type 7F and 15B/C. Total of 8 isolates (38.1%) were found to be nontypeable isolates. Multiplex PCR was successfully identified different types of CPS. Development of Multiplex PCR could help in diagnosing and identifying capsular type of S. pneumoniae simultaneously.
Keywords: Streptococcus pneumoniae, Capsular Polysaccharides (CPS), Multiplex PCR, Positive Control, Pneumococcal vaccine
INTRODUCTION
Pneumonia is still a threatening problem in the world, especially in developing countries. It can cause largely by bacteria, beside virus and fungal. One of bacteria responsible in pneumonia is Streptococcus pneumoniae. S. pneumoniae or Pneumococcus is a gram-positive coccus that is causing pneumococcal disease mainly in children aged less than five years and in the elderly. The spectrum of diseases caused by this bacterium can be classified into non-invasive (mucosa) and invasive pneumococcal diseases. Sinusitis, otitis media, and respiratory tract infections (pneumonia) are non-invasive diseases, while
meningitis and sepsis are known as Invasive Pneumococcal Diseases (IPD) (Drijkoningen and Rohde 2013). WHO reported that almost 1 million IPD cases occur among children younger than 5 years (Rudan et al, 2008). Previous study reported that approximately 100-600/100.000 IPD cases occurred in developing country (Jauneikaite et al, 2012; Rudan et al, 2008). Most of S. pneumoniae infections are asymptomatic and initiated as healthy carrier in nasopharynx among childhood age (Adetifa et al, 2012). S. pneumoniae has many virulence factors. One of the major virulence factors is capsular polysaccharide (CPS) (Jedrzejas, 2001; Mitchell and Mitchell, 2010; Geno et al,
2015). There are 93 serotypes of S. pneumoniae are known based on CPS types, and 15 of them are involved in IPD (Lin et al, 2006; Geno et al, 2015; Drijkoningen and Rohde, 2013). CPS is used for pneumococcal vaccine to prevent pneumococcal infections. Types of pneumococcal conjugate vaccines (PCV), especially for children, include polysaccharide antigens against 7 (7-valent), 10 (10-valent) or 13 (13-valent) serotypes of CPS. Vaccine serotypes are categorized based on the following vaccine preparations: 7 valent — 4, 6B, 9V, 14, 18C, 19F, and 23F, 10 valent — 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, and 23F; and 13 valent — 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F (Adetifa et al, 2012; Geno et al, 2015; Hung et al, 2013). Since CPS is the major component in Pneumococcal Vaccine, several studies have been conducted to figure out the CPS types spreading, including in Indonesia. Several studies of healthy carrier of S. pneumoniae have been conducted in Indonesia. Study conducted in Lombok, West Nusa Tenggara reported the dominant types of S. pneumoniae CPS were 6, 23, 15, 33 dan 12 (Soewignjo et al.,, 2001), whereas in Jakarta was 7F (Yuliarti et al.,, 2012), and in Central Java were 6A/B and 15B/C (Farida et al.,, 2014). Different methods have been used to determine CPS types. Two former studies used serology based, however, the latter studies performed PCR (molecular method) to identify CPS types (Soewignjo et al.,, 2001; Yuliarti et al.,, 2012; Farida et al.,, 2014). Molecular methods, named PCR, nowadays is promising method for typing of CPS because it directly detects or amplifies the CPS type gene specifically, is relatively easy to get the reagents and to perform, and can accommodate amplification of several types simultaneously (Multiplex PCR) (Rubin et al, 2004; Pai et al, 2006; Saha et al, 2008; Yun et al, 2011). Since no data have
reported CPS types of S. pneumoniae in Bali, and the molecular method for simultaneous detection of CPS type have not been yet optimized in our laboratory; therefore, this study aimed to determine CPS types of S. pneumoniae isolates in Clinical
Microbiology Laboratory, Sanglah General Hospital, Denpasar, Bali using Multiplex PCR.
MATERIALS AND METHODS Bacterial strains, medium and growth conditions
Twenty-one glycerol stock isolates of S. pneumoniae that have been stored properly at -80°C were cultured on 5% sheep blood agar plate and incubated aerobically at 35±2°C for 18-24h. The isolates were isolated from blood (11/52.4%), sputum (5/23.8%), and other clinical specimens (5/23.8%) during 2012-2015. Identification of S. pneumoniae was based on colony characteristics and sensitivity of the isolates against a 5 μg optochin disk (Oxoid) that shown as inhibition zone of the isolates more than 16 mm.
Bacterial Genomic DNA Isolation
Isolation of S. pneumoniae genomic DNA from colonies used Roche High Pure PCR Template Isolation Kit (Roche Life Science, Indianapolis, USA). Briefly, S. pneumoniae colonies were harvested and suspended in 200 μl Phosphate Buffered Saline pH 7.3. Bacterial suspension was then subjected to DNA isolation steps based on manufacturer’s recommendation. Final elution volume was 50 μl.
PCR for pneumolysin gene (ply)
Presence of pneumolysin gene (ply) was detected by PCR. PCR mixture used for ply amplification was Go Taq® Green Master Mix (Promega, Madison, USA). Specific primer pairs for ply were 5’-ATTTCTGTAACAGCTACCAACGA-3’ (forward) and 5’-
GAATTCCCTGTCTTTTCAAAGTC-3’ (reverse) (Saleem et al, 2012). Concentration of primer was 0.5 μM each. PCR cycle involved pre denaturation at 94°C for 2 min; 35 cycles of denaturation at 94°C for 30 s, annealing primer at 54°C for 30 s and extension at 72°C for 1 min; and final extension at 72°C for 5 min (iCycler, Biorad thermal cycler). Amplicons were electrophoresed on 1.5 % agarose gel in TBE buffer at 60 volt and for 35 min. DNA was visualized with GelRed™ Nucleic Acid Gel Stain (Biotium, Hayward, CA 94545) and captured (Gel Doc, Biorad). Positive result was shown as a 348 bp-band.
PCR for Capsular Polysaccharide (CPS) typing
PCR for capsular typing was conducted in two different methods. Firstly, Uniplex PCR was performed to determine the CPS type individually, and then continued with Multiplex PCR to determine the CPS type simultaneously. Go Taq® Green Master Mix (Promega, Madison, USA) was used for Uniplex PCR, while Kapa 2G Fast ReadyMix PCR Kit with dye (Kapa Biosystems) was for Multiplex PCR. Uniplex PCR for CPS typing used primer final concentration 0.3 μM each. PCR cycle was initiated with pre denaturation at 94°C for 2 min; 30 cycles of denaturation at 94°C for 1 min, annealing primer at 54°C for 1 min and extension at 72°C for 1 min; and final extension at 72°C for 5 min (iCycler, Biorad thermal cycler). Protocols of Multiplex PCR
were described in previous study with modification (Pai et al, 2006). Briefly, the cycle involved pre denaturation at 95°C for 2 min, continued with 35 cycles of denaturation at 95°C for 1 min, annealing primer at 54°C for 1 min and extension at 72°C for 1 min, and final extension at 72°C for 5 min (iCycler, Biorad thermal cycler). PCR for CPS types was performed with primers listed in Table 1, based on previous study (Pai et al, 2006). Amplicons were then run on 1 % agarose gel in TBE buffer at 60 volt, and for 35 min. DNA was visualized with GelRed™ Nucleic Acid Gel Stain (Biotium, Hayward, CA 94545) and then captured with gel documentation (Gel Doc, Biorad).
RESULTS AND DISCUSSION
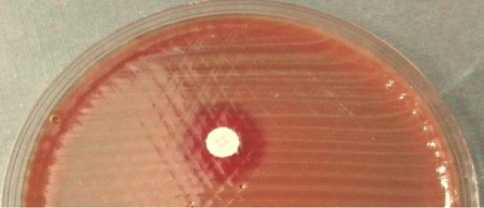
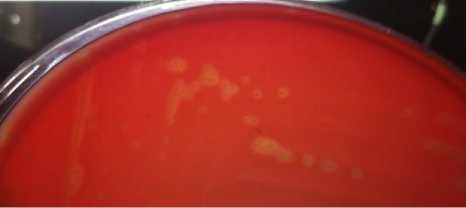
All glycerol stocks of S. pneumoniae were successfully cultured on 5% sheep blood agar plates. The colonies showed pinpoint colonies surrounded with greenish zone referred as alpha hemolysis type (Figure 1a). Furthermore, the optochin test of the colonies showed inhibition zone more than 16 mm (susceptible). These findings concluded that all of the isolates were S. pneumoniae phenotypically. PCR was then conducted to confirm the presence of pneumolysin gene (ply). All of the isolates had ply gene confirming that all isolates were S. pneumoniae based on its genotype.
CPS typing was then performed by PCR. Firstly, Uniplex PCR was performed to amplify CPS gene individually. This method was conducted in order to ensure and optimize that the PCR system was in optimal conditions and to develop positive control (in-house positive control) for subsequent Multiplex PCR. The result of Uniplex PCR showed that type 19F was the predominant type (7 isolates (66.7%)); while 2 isolates
(9.5%) were positive for each type 23F and also for type 6A/B; and only 1 isolate (4.8%) was found for each type 7F and 15B/C. Total of 8 isolates (38.1%) were found to be nontypeable isolates (Figure 3a and b). Many studies have been conducted to determine the CPS types, both in hospital based or community based. Study in Malaysia showed that most of S. pneumoniae carrier have serotypes 19F dan 23F (Le et al, 2011). Studies of healthy carrier of S. pneumoniae also have been conducted in Indonesia. As previously mentioned, study in Lombok, West Nusa Tenggara reported the dominant types of S. pneumoniae CPS were 6, 23, 15, 33 dan 12 (Soewignjo et al, 2001), whereas in Jakarta was type 7F (Yuliarti et al, 2012). Different data was also found in Central Java, which reported predominant types found in this area were types 6A/B and 15B/C (Farida et al, 2014). Although there are variations of the predominant circulating CPS types in several areas in Indonesia, including in this study, all of S. pneumoniae CPS types circulated are still vaccine-type CPS.
Two different groups of Multiplex PCR were developed in this study considering the band size of each type. Multiplex PCR of Group I involved 23F, 6A/B, and 7F, while Group II was 19F and 15 B/C. Positive controls for the two multiplex PCR were developed by mixing the amplicons from the CPS Uniplex PCR, which was adjusted based on Multiplex PCR Group.
Amplification of the positive control using Multiplex PCR was successfully
performed; suggesting the control positive developed in this study can be used for Multiplex PCR for amplifying the CPS type. Furthermore, the two multiplex PCR were successfully amplified all of CPS type precisely as CPS Uniplex PCR shown (data not shown). Previous study of CPS typing, either serology or molecular based, both of methods could detect CPS types.
However, recently, molecular method is widely used for typing because of relatively low cost, ease of reagents handling, and direct detection of certain gene (Rubin et al, 2004; Pai et al, 2006; Saha et al, 2008; Yun et al, 2011).
Taking together, the results suggested that predominant CPS types circulated among clinical isolates of S. pneumoniae in Sanglah General Hospital were somewhat different with those in other areas in Indonesia. In addition, development of Multiplex PCR could help in diagnosing and identifying capsular type of S. pneumoniae simultaneously; therefore, will help in mapping the
predominant CPS type in the community. The limitations of this study are small number of sample included in this study, and the nontypable isolates still need to be further identified. Future study for CPS typing especially in community or among healthy carrier will be helpful in determining circulation of vaccine-type of S. pneumoniae; therefore, could predict the successful rate of pneumococcal vaccine in Bali.
Table 1. Primer used for Capsular Typing of S. pneumoniae (Pai et al.,, 2006)
|
No. |
Nama Primer |
Sekuens Primer (5'÷ 3’) |
Bp |
|
1. |
6A/B-f |
AAT TTG TAT TTT ATT CAT GCC TAT ATC TGG |
250 |
|
6A/B-r |
TTA GCG GAG ATA ATT TAA AAT GAT GAC TA | ||
|
2. |
7F-f |
CCT ACG GGA GGA TAT AAA ATT ATT TTT GAG |
826 |
|
7F-r |
CAA ATA CAC CAC TAT AGG CTG TTG AGA CTA AC | ||
|
3. |
7C-f |
CTA TCT CAG TCA TCT ATT GTT AAA GTT TAC GAC GGG A |
260 |
|
7C-r |
GAA CAT AGA TGT TGA GAC ATC TTT TGT AAT TTC | ||
|
4. |
12F-f |
GCA ACA AAC GGC GTG AAA GTA GTT G |
376 |
|
12F-r |
CAA GAT GAA TAT CAC TAC CAA TAA CAA AAC | ||
|
5. |
15A-f |
ATT AGT ACA GCT GCT GGA ATA TCT CTT C |
436 |
|
15A-r |
GAT CTA GTG AAC GTA CTA TTC CAA AC | ||
|
6. |
15B/C-f |
TTG GAA TTT TTT AAT TAG TGG CTT ACC TA |
496 |
|
15B/C-r |
CAT CCG CTT ATT AAT TGA AGT AAT CTG AAC C | ||
|
7. |
19F-f |
GTT AAG ATT GCT GAT CGA TTA ATT GAT ATC C |
304 |
|
19F-r |
GTA ATA TGT CTT TAG GGC GTT TAT GGC GAT AG | ||
|
8. |
23F-f |
GTA ACA GTT GCT GTA GAG GGA ATT GGC TTT TC |
384 |
|
23F-r |
CAC AAC ACC TAA CAC ACG ATG GCT ATA TGA TTC | ||
|
9. |
33F-f |
GAA GGC AAT CAA TGT GAT TGT GTC GCG |
338 |
|
33F-r |
CTT CAA AAT GAA GAT TAT AGT ACC CTT CTA C |
a
b
Fig. 1. Colonies of S. pneumoniae on 5% sheep blood agar plate (BAP). The colonies’s characteristics are pin-point colonies surrounded by greenish zone referred as alpha hemolytic (a). Optochin sensitivity test for S. pneumoniae identification. S. pneumoniae is an optochin-susceptible bacterium. The diameter of inhibition zone around a 5 μg-optochin disk is more than 16 mm (b). ACKNOWLEDGMENTS
This work was financially supported by Hibah Penelitian Unggulan Udayana Lembaga Penelitian dan Pengabdian kepada Masyarakat (LPPM) Udayana University, Bali, Indonesia under Grant No. 246-329/UN14.2/PNL.01.03.00/2015. High
appreciation is dedicated to Putu Yuliandari,
M.D. (Clinical Microbiology Laboratory, Faculty of Medicine staff) for technical and administration support. We also thank Wahyu Hidayati and Senshi Septia (Molecular Biology Laboratory staff), and Ni Wayan Nilawati (Clinical Microbiology Laboratory Sanglah General Hospital staff) for their technical supports.
REFERENCES
Adetifa IMO, Antonio M, Okoromah CAN, Ebruke C, Inem V, Nsekpong D, Bojang A, and Adegbola RA. 2012. Pre-vaccination nasopharyngeal
pneumococcal carriage in a Nigerian population: epidemiology and
population biology. PLoS ONE. 7: 30548.
Drijkoningen JJC and Rohde GGU. 2013. Pneumococcal infections in adults. Clin. Microbiol. Infect. 20: 45-51.
Farida F, Saverin JA, Gasem MH, Keuter M, Wahyono H, Broek PVD, Hermans PWM, and Verbrugh HA. 2014. Nasopharyngeal carriage of Streptococcus pneumoniae in pneumonia-prone age groups in Semarang, Java Island, Indonesia. PLoS ONE. 9: 87431.
Geno, K.A, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, and Nahm MH. 2015. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 28: 871–899.
Hung IFN, Tantawichien T, Tsai YH, Patil S, and Zotomayor R. 2013. Regional epidemiology of invasive
pneumococcal disease in Asian adults: epidemiology, disease
burden, serotype distribution, and antimicrobial resistance patterns and prevention. Int. J. Infect. Dis. 17: 364–373.
Jauneikaite E, Jefferies JM, Hibberd ML, and Clarke SC. 2012. Prevalence of
Streptococcus pneumoniae serotypes causing invasive and non-invasive disease in South East Asia: A review. Vaccine. 30: 3503–3514.
Jedrzejas M.J. 2001. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 65: 187–207.
Le CF, Palanisamy NK, Yusof MYM, and Sekaran SD. 2011. Capsular serotype and antibiotic resistance of Streptococcus pneumoniae isolates in Malaysia. PLoS ONE. 6: 19547.
Mitchell A and Mitchell TJ. 2010. Streptococcus pneumoniae: virulence factors and variation. Clin. Microbiol. Infect. 16: 411-418
Pai R, Gertz RE, and Beall B. 2006. Sequential Multiplex PCR Approach for Determining Capsular Serotypes of Streptococcus pneumoniae Isolates. J. Clin. Microbiol.. 44: 124– 131.
Rubin LG and Rizvi A. 2004. PCR-based assays for detection of Streptococcus pneumoniae serotypes 3, 14, 19F and 23F in respiratory specimens. J. Med. Microbiol. 53: 595–602.
Rudan I, Pinto CB, Biloglav Z, Mulholland K, and Campbell H. 2008. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 86: 408– 416.
Saha SK, Darmstadt GL, Baqui AH, Hossain B, Islam M, Foster D, Emran HA, Naheed A, Arifeen SE, Luby SP, Santosham M, and Crook D. 2008. Identification of serotype in culture negative pneumococcal meningitis using sequential
multiplex PCR: implication for surveillance and vaccine design. PLoS ONE. 3: 3576.
Saleem M, Naz M, Waris A, Muneer B, and Khurshid R. 2012. Screening of pneumococcal pneumonia by amplification of pneumolysin gene in children visiting hospitals in Lahore,
Pakistan. Iran. J. Pediatr. 22: 524– 530.
Soewignjo S, Gessner BD, Sutanto A, Steinhoft M, Prijanto M, Nelson C, Widjaya A, and Arjoso S. 2001. Streptococcus pneumoniae
nasopharyngeal carriage prevalence, serotype distribution, and resistance patterns among children on Lombok Island, Indonesia. Clin. Infect. Dis. 32: 1039–1043.
KW, Chao EY, Hong KB, Choi EH, and Lee HJ. 2011. Streptococcus pneumoniae Type Determination by Multiplex Polymerase Chain Reaction. J. Korean. Med. Sci. 26: 971–978.
99 • asia oceania biosciences and biotechnology consortium
Discussion and feedback