UTILIZATION OF RHIZOSPHERE FUNGI TO CONTROL Fusarium oxysporum f.sp. capsici IN VITRO
on
UTILIZATION OF RHIZOSPHERE FUNGI TO CONTROL Fusarium oxysporum f.sp. capsici IN VITRO
I Made Sudarma1*, Ni Made Puspawati1, Ni Wayan Suniti1, I Nyoman Wijaya2 and I Gusti Ngurah Bagus2
1Laboratory staff of Plant Pathology, Department of Agroetechnology, Agriculture Faculty of Udayana University
2Laboratory staff of Plant Entomology, Department of Agroetechnology, Agriculture Faculty of Udayana University
*Corresponding author : sudarma_made@ymail.com
ABSTRACT
Fusarium caused wilt disease in chilli pepper and destroyed some farmer crops. Results of preliminary research has been discovered that the disease caused by Fusarium oxysporum f.sp. capsici. The alternative environmental friendly method is to find antagonist microbes which is located in the rhizosphere of healthy pepper plants. This study aims to find out potentially antagonistic fungi to control Fusarium wilt disease on pepper plants. The fungi were isolated by soil dilution method or viable plate count method on Potato Dextrose Agar medium with antibiotic Livoplaxacin (25%, w/v). Rhizosphere fungi from healthy pepper plants had been identified. A total of 63 spesies belong to 4 genera included Penicillium (45 species), Aspergillus (6 species), Trichoderma (9 species) and Candida (3 species). The highest percentage of distribution of rhizosphere fungi are P. digitatum (47.63%), P. expansum (19,05%), T. harzianum (9,53%), A. nidulans, A. niger, Penicillium sp., Candida albicans, and T. vitrens i.e 4,76% respectively. All of rhizosphere fungi colonies were found to inhibit Fusarium oxysporum f.sp. capsici in vitro. The best inhibition was found in Aspergillus niger at 88.89 ± 2.2% followed by A. nidulans of 85,56 ± 1,6 %, T. harzianum at 84,45 ± 1,58% , and T. virens by 83,33 ± 1,2%, five days after inoculation. All of them have a very high inhibition criteria.
Keywords: Rhizosphere fungi, Fusarium oxysporum f.sp. capsici, inhibiting ability, persentage contribution, and antagonistic.
INTRODUCTION
Soil fungi, especially living in the rhizosphere was one of all microorganisms that plays an important role in soil fertility and plant health. Soil was a harbor that has a very high biological diversity and were not yet fully capable studied (Chandrashekar et al.,, 2014; Berg et al.,, 2015).
Rhizosphere role was inseparable from the issuance exudates. Root exudates of some plants such as peppers (Capsicum annuum L.) issued detected 12 amino acid and 7 of sugar. On resistant cultivars root exudates containing mithionine, d-1-β phenylalanine, citrulline and D-xylose. Root
exudates that originated from resistant varieties can inhibit germination of pathogen (Fusarium oxysporum f.sp. capsici), but susceptible varieties increase spore germination same pathogens. Germination of fungal spore antagonistic (Trichoderma viride and Aspergillus sydowi) is also influenced by root exudates of resistant and susceptible varieties, but the effect is different ((Naqvi and Chauhan, 1980).
The fungal rhizosphere that antagonistic gave contribution to controlling soil borne pathogen. That was such as Penicillium spp., Aspergillus spp., Gliocladium sp., and Trichoderma spp., be able to suppress mycelium of F. oxysporum
f.sp. cubense (cause Fusarium wilt disease of banana) in vitro (Sudarma and Suprapta, 2011). Some interaction happened in rhizosphere related with soil borne pathogen suppress. The interaction included competition, parasitism, and induced plant resistant (Wipps, 2001).
Plant root disease with wilt symptom in pepper plant can caused by several fungus such as Phytophthora capsici (Sranghellini et al.,, 1996), and Fusarium wilt disease caused by F. oxysporum f.sp. capsici (Wongpla and Lomthalsong, 2010). Pepper plant that attacked by two pathogen like mentioned above found similar symptom, where the plant wilt result the vascular tissue plugged, then water and nutrient not be able transported to apart of plant from root and stem. Result work of Sudarma and Puspawati (2013) (unpublish data) has identification the pathogen of wilt disease in plant pepper, at Banjarangkan district, Klungkung regency, was Fusarium oxysporum f,sp. capsici with disease incidence was 76,67%. Disease epidemic of Fusarium wilt in plant pepper was 0.44 per unit per day, then decreased 0.23, 0.12 and 0.11 per unit per day. Therefore, potential of fungal antagonistic that originated from soil rhizosphere (suppressive soil) should be used as biological control agent to controlling soil borne pathogen such as pathogen that cause Fusarium disease in plant pepper.
MATERIALS AND METHOD
Soil samples
Soil samples taken from rhizosphere of pepper plant healthy with diagonal method for one field, and replicated three times. For each hole for a pepper plant healthy was taken 100 g soil, and mixed before taking place in plastic bag, than put in an ice box.
Before the analyzed soil samples were placed in refrigerator for 24 hours.
Isolation of fungal rhizosphere
Each soil samples was taken 10 grams of soil was diluted with 90 ml of sterile water, dilution was continued 10-3- 10-6. One milliliter was placed in a Petri dish had previously been filled with potato dextrose agar (PDA) medium and supplemented with antibiotics (antibacterial) levoplaxasin 250 mg/l (w/v). Colonies of rhizosphere fungi will grow after two days, then count the number colonies by colony forming unit (cfu). Furthermore, each colony purified and transferred to a new Petri dish.
The percentage of distribution
The percentage contribution can be calculated as follows, total number of colony forming unit (cfu) of a particular species divided by the total number of cfu entire species times 100% (Chandrashekar et al.,, 2014). The percentage contribution may illustrate that there are certain species that dominate and contribute in rhizosphere of pepper plant healthy, it can be seen with the highest percentage value contribution.
Inhibition test of fungal rhizosphere
Each rhizosphere fungal tested for inhibitory against the growth of F. oxysporum f.sp. capsici with a dual culture technique. Percent inhibition can be calculated using the following formula (Dolar, 2001; Jayalal and Adikaram, 2007; Mojica-Martin et al.,, 2008):
A – B
% inhibition = x 100
A
A = Diameter of Fusarium oxysporum f.sp. capsici colony in the control (mm)
B = Diameter of rhizosphere fungal colony in treated (mm).
Inhibition ability of rhizosphere fungal against pathogen was calculated every day until the control (F. oxysporum f.sp. capsici, single culture) and observation halted until control have met Petri dishes. Antagonistic inhibition mechanism against pathogen can be known whether it competition or antibiosis by observing the presence and absence inhibition zones in Petri dish. If the any clear zone (zone of inhibition) mean inhibition by antibiosis mechanism. Inhibition criteria can be grouped as follows: inhibition of 0-20% = very low, 21-40% = lower, 41-60% = moderate, 61-80% = high, and 81-100% = very high.
Identification of rhizosphere fungal of pepper plant healthy
Rhizosphere fungal have been successfully purified and gown in culture medium PDA in Petri dish, subsequently indentified microscopic morphology included shape and color of conidia (spores), conidiophores, hyphae structures. The fungal
rhizosphere documented by using the tool OPTILAB that relate directly to a laptop. The observation was matched with a compatible reference, such as Samson et al.,, 1981; Pitt dan Hocking, 1997; Barnett dan Hunter, 1998; dan Indrawati et al.,, 1999. Diba et al.,, 2007; Rahman et al.,, 2011; Samson et al.,, 2011).
RESULTS AND DISCUSSION
Identification of fungal rhizosphere
A total 63 isolates of fungal origin rhizosphere of pepper plant healthy have been identified which are included in the four genera such as Aspergillus, Candida, Penicillium and Trichoderma. In the Aspergillus genera were found six species such as A. nidulans and A niger three species, respectively. Same with genera of Candida only three species, namely C. albicans. In the genera of Penicillium found 45 species (30 species of P. digitatum, 12 species of P. expansum, and 3 species of Penicillium sp). In the genera of Trichoderma was found 9 species (six species of T. harzianum, and three species of T. virens) (Table 1).
Table 1. The number of species, colonies and percentage contribution of rhizosphere fungal of pepper plant healthy
|
No. |
Spesies of rhizosphere fungal |
Number of colony (CFU/g of soil) x 103 |
% contribution |
|
1 |
Aspergillus nidulans |
3 |
4.76 |
|
2. |
Aspergillus niger |
3 |
4.76 |
|
3 |
Candida albican |
3 |
4.76 |
|
4 |
Penicillium digitatum |
30 |
47.62 |
|
5. |
Penicillium expansum |
12 |
19.05 |
|
6 |
Penicillium sp. |
3 |
4.76 |
|
7 |
Trichoderma harzianum |
6 |
9.52 |
|
8 |
Trichoderma virens |
3 |
4.76 |
|
Total |
63 |
100 |
Contribution percentage of rhizosphere fungal found was highest 47.62% by P. digitatum, followed by P. expansum was 19.05%, 9.52% of T. harzianum, and A. nidulans, A. niger, Candida albican, and T. virens were 4.76%, respectively (Table 1)
Aspergillus genera has conidiophores upright, simple, terminating in a glubosa or clavate swelling, bearing phialides at the apex or radiating from the apex or the entire surface; conidia (phialospores) 1-celled, globose, often variously colored in mass, in dry basipetal chains (Barnett dan Hunter, 1998; Gautam and Bhadauria, 2012). Aspergillus genera consists of hundreds od species of mold that was faound throughout the world. First discovered in 1729 by biologist Pier Antonio Micheli of Italy. Aspergillus name was also the name of the structure formation of asexual spores generally to all species of Aspergillus. About a third of species are also known to have a sexual stage. Aspergillus can be classified as follows : kingdom of Fungi, Division of Ascomycota, classes of Eurotiomycetes, Order of Eurotiales, family of Trichocomaceae, and the genera of Aspergillus (Bennett, 2010).
Candida albicans, can grow in at least three different morphologies; yeast, pseudohyphae and hyphae (Sudbery et al.,, 2004). Candida albicans is a fungus species are pathogenic to humans from devisio Deuteromycota . This fungus is the cause oppotunistic infection called candidiasis of the skin, mucosa, and organs in humans. Some characteristics of this species are eggshaped ( ovoid ) or spherical with a diameter of 3-5 μm and can produce pseudohyphae. Rose (1990) states that Candida albicans has two types of morphology, namely forms such as yeast and hyphae form. Additionally, phenotype or appearance of these microorganisms can also be changed from
white and flat to become wrinkle irregular, star-shaped, circular, shapes such as coffee, and opaque . This fungi have the ability to attach to host cells and colonize.
Candida albicans was included in the phyllum Ascomycota, upphyllum of Saccharomycetes, class of Saccharomycetes, order of Sacharomycetales, family of Sachasromycetadae, genera of Candida, and species of Candida albicans ( CP Robin ) Berkhout. 1923. synonyms: Candida stellatitreae, and Oidium albicans (Sudbery, 2011).
Colonies of Penicillium sp. usually green, sometimes white, mostly have conidiophores. Single conidiophores (mononematus) or compound (synematous), consisting of a single trunk dividing some phialide (simple/monoverticillata). Phialide was a structure that sustains conidia, cylindrical basal part narrowed neck, or lancoelate (approximately partially embedded in the basal part of the end pieces). Conidia form long chains, divergent or column, globular, elliptical or fusiform, transparent or greenish, with walls smooth or bumpy (Gam et al.,, 1987). Conidia form long chains, divergent or column, globular, elliptical or fusiform, transparent or greenish, with walls smooth or bumpy (Figure 3) (Alexander, 1930).
Trichoderma spp. was a cosmopolitan fungus , often exist in all types of soil, manure and decaying plant tissue. Dominance in the ground equipped with a diversity of metabolic capabilities and competitive nature aggressive. These characteristics make this fungus on wood decomposers significant and herbs. Trichoderma spp. also able to degrade waste relatively quickly without removing the stench (Rahman et al.,, 2011). Trichoderma is included in the kingdom Fungi, Ascomycota devisio, subdevisio
Pezizomycotina, class of Sordariomycetes, order of Hypocreales, family of Hypocreaceae familia, and genera of Hypocrea (perfect stage) or Trichoderma (imperfect stage) ( Druzhinina et al.,, 2005).
Inhibition ability test of fungal rhizosphere
Inhibition ability of fungal rhizosphere against F. oxysporum f.sp. capsici was found highest in Aspergillus niger by 88.89 ± 2.2%, followed by A. nidulans of 85.56 ± 1.6%, Trichoderma harzianum of 84.45 ± 1.58%, P. expansum of 84.44 % ± 2.22%, T. virens of 83.33 ± 1.2%, P. digitatum of 79.35 ± 14.29%, Penicillium sp. of 78.89 ± 1.3%, and Candida albicans by 69 ± 1.3% at age 6 days after inoculation (Table 2). Inhibitory mechanism fungi in the rhizosphere against pathogen were of antibiotics and competition. Antibiosis mechanism that found in the Petri dish there was a yellowish translucent zone issued by antagonist fungi ( Aspergillus nidulans, and Penicillium sp.) (Figure 2).
Others show the mechanism of inhibition of the competition, both space and nutrient competition. Fungal antagonists were found to justify the theory that the suppressive soil (healthy plant habitats) will contain many types and density of fungal antagonist which protects the roots of healthy plants from pathogens infection in the rhizosphere. This fungus has the potential to be developed and applied in vivo ( Sudarma and Suprapta, 2011).
Aspergillus spp. has been tried in vitro, can suppress the growth of P. palmivora (cause fruit rot disease of cocoa). Aspergillus niger has the highest inhibition than both the other fungus (A. fumigatus and A. repens ), which amounted to 54% (Adebola and Amadi, 2010). Aspergillus
niger can improve biological control of inoculant bacteria ( P. fluorescens ) against the disease of root knot nematodes, as well as against Fusarium spp. (Siddiqui et al.,, 2004; Dawar et al.,, 2008). Aspergillus nidulans can be antagonistic to Colletotrichum gloeosporioides (causes antraknose in vanilla plant). The result of Fakhrunnisa et al., ( 2006) research found that A. niger, can inhibit the growth of Fusarium spp. through antibiosis mechanism in vitro. Bosah et al., (2010) has also been found that Aspergillus spp. can inhibit the growth of pathogenic fungi Sclerotium rolfsii with inhibition of 73.12 to 88.35%. The process of inhibition caused by Aspergillus spp. produce the enzyme chitinase and β-1 , 3-glucanase (Laminarinase) that has the ability to break down the components of fungal cell walls of pathogens such as chitin and β-1,3- glucan.
Candida albicans was a form of yeast that is found in all humans. This fungus was usually harmless, but if the population exceeds controls. Candida albicans reproduce themselves by forming buds that will continue pseudohyphae elongated shape. Pseudohyphae were formed with many groups blastospore round or oval around the septum.
In some strains, blastospores large, round or like bottles, in small amount these cells can develop into thick-walled chlamydospores and a diameter of about 8-12 µm. Morphology of C. albicans colonies on solid medium in Sabouraud Dextrose Agar, generally round in shape with a slightly convex surface, smooth, slippery and sometimes a little convoluted, especially in colonies that have been older. Age culture affect the small colony.
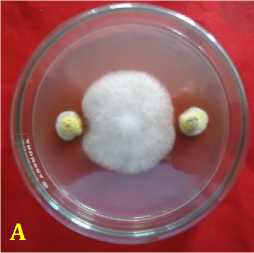
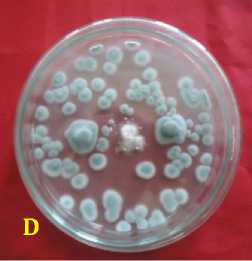
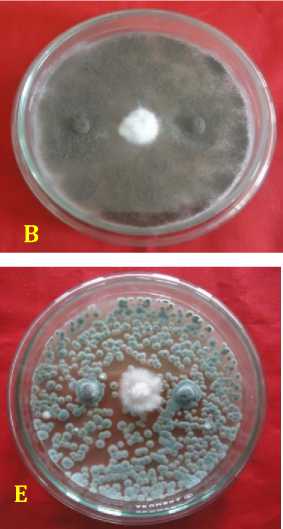
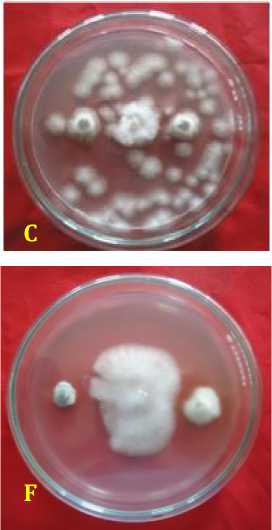
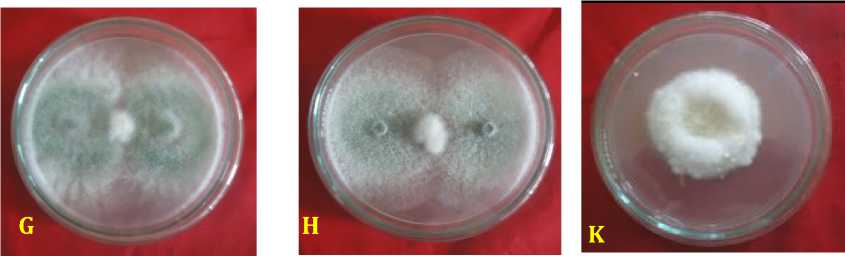
Fig. 2. Inhibition ability of fungal rhizosphere against Fusarium oxysporum f.sp. capsici in vitro. (A = A. nidulans, B = A. niger, C = Candida albicans, D = P. digitatum, E = P. expansum, F = Pinicillium sp., G = T. harzianum, H = T. virens, dan K = kontrol or F. oxysporum f.sp. capsici), 3 days after inoculation.
Colonies yellowish white color and smelled like sour aroma tape. In a liquid medium such as glucose yeast extract peptone, Candida albicans grows at the base of the tube (Jha et al.,, 2006). Candida albicans is a dimorphic fungus because of its ability to grow in two different forms, namely as a stem cell will develop into blastospora and produce sprouts that will form a pseudohyphae . Yeast cells (blastospore) is round, oblong or oval with a size of 2-5 x 3-6 μm up to 2-5.5 x 5-28 μm (Tsafrir, 2015).
Penicillium sp. have been tried as microbial antagonists against Sclerotium rolfsii which is a soil borne pathogen that damages more than 500 species of plants, but the most inhibitory power lower than Trichoderma sp. and Aspergillus sp. which amounted to 46.24-56.98% (Bosah et al.,, 2010). Haggag and Mohamed (2007), states that Penicillium sp. can antagonistic through a mechanism that is issued several alkaloid compounds such as agroklavine and ergometrine which has anti-fungal properties against Botrytis cinerea, Fusarium solani and
Alternaria tenius. Idris et al.,, (2008 ) have found Penicillium sp. as microbial antagonists against Ganoderma sp. cause disease in plants Palma. Penicillium sp. has also been tried in controlling the Lanas disease in tobacco plants caused by Phytophthora parasitica var. nicotianae (Roeswitawati, 2007). Mechanism of action of penicillin that has activity against the synthesis of peptidoglycan, which causes cell lysis and death. They inhibit one of the stages required for cross- bonding peptidoglycan, transpeptidation, because of similarities between molecules streriochemical penicillin and D-Ala-D-Ala dipeptide. This enzyme is located in the outer regions of the cytoplasmic membrane: penicillin binding proteins (penicillin-binding protein/PBP) (Bryskier, 2005).
Trichoderma was also known as mycoparasit that can grow and take nutrients in pathogens, so that pathogens can not thrive in soil. Trichoderma harzianum has the ability antagonist best compared with other antagonist microbes, such as Bacillus thuringiensis, Rhizobium meliloti and A. niger to control root rot sunflower crop (Dawar et al.,, 2008). The mechanism of inhibition of Trichoderma against soil borne pathogens, namely: (1) generate the enzyme chitinase, β-1,3-glucanase (Katatny et al.,, 2000), β-1,4 glucanase and lipase compound that can break down chitin, glucan and fats the cell walls of pathogens (Benitez et al.,, 2004; Vinale et al., (2008); (2) mycoparasitism (Howell, 2003); (3) antibiosis to produce antibiotic 6-pentyl-a-pyrone (6pp), heptilidic acid and peptaibol (Barea et al.,, 2005; Vinale et al.,, 2008); (4) competition for nutrients and space (Lo, 1998; Benitez et al.,, 2004); (5) the root colonization (Harman et al.,, 2004; Vinale et al.,, 2008); induce local and systemic resistance (Harman, 2006).
CONCLUSION
Rhizosphere fungal origin from pepper plant healthy were able to be identified was four genus which consists of: Penicillium genera includes 30 species of Penicillium digitatum, three Penicillium sp., and 12 species of P. expansum; six genera of Aspergillus which consists of each of the three species of A. nidulans and A. niger; three genera of Trichoderma which consists of six species each of the three species of T. harzianum and T. virens three species; and three species of the Candida albicans. The prevalence of rhizosphere fungal as follows: P. digitatum highest of 47.63%, subsequently P. expansum by 19.05%, 9.53% of T.
harzianum, A. nidulans, A. niger, Penicillium sp., Candida albicans and T. vitrens each by 4.76 %. All rhizosphere fungal isolates were found to inhibit pathogen (Fusarium oxysporum f.sp. capsici) in vitro, very high inhibition was found in Aspergillus niger, hereinafter Aspergillus nidulans, Penicillium expansum, Trichoderma harzianum, and Trichoderma virens, with a very high inhibition criteria.
ACKNOWLEDGEMENTS
Authors wish to thank to the Rector of Udayana University for their assistance and the opportunity given so that research can be resolved. Dean of Faculty of Agriculture, Udayana University, and Chairman of Institute for Research and Community Service Udayana University, for the their help and cooperation so that research can be funded to completions.
REFFERENCES
Adebola, M.O. and J.E. Amadi, 2010.
Screening three Aspergillus species for antagonistic activities against the cocoa black pod organism
(Phytophthora palmivora).
Agriculture and Biology Journal of North America 1(3): 362-365.
Barea, J.M., M.J. Poso, R. Azcon and C.A. Aguilar. 2005. Microbial co-operation in the rhizosphere. Journal of Experimental Botany, 56(417): 17611778.
Barnett, H.L. and B.B. Hunter. 1998. Illustrated Genera of Imperfect Fungi. APS Press. The American Phytopathological Sociey. St Paul, Minnesota.
Benıtez, T., A.M. Rincon, M.C. Limon, and A.C. Codon. 2004. Biocontrol mechanisms of Trichoderma strains. International Microbiology 7: 249– 260.
Bennett J.W. 2010. An Overview of the Genus Aspergillus. Aspergillus:
Molecular Biology and Genomics. Caister Academic Press.
Berg, G., C. Zachow, J. Lottmann, M. Gotz, R. Costa and K. Smalla. 2015. Impact of Plant Species and Site on Rhizosphere-Associated Fungi
Antagonistic to Verticillium dahlia Kleb. American Society of Microbiology. Journal ASM.org71(8) 4203-4213.
Bosah, O., C.A. Igeleke and V.I. Omorusi. 2010. In Vitro Microbial Control of Pathogenic Sclerotium rolfsii. Int. J. Agric. Biol 12: 474–476.
Bosah, O., C.A. Igeleke and V.I. Omorusi. 2010. In Vitro Microbial Control of Pathogenic Sclerotium rolfsii. Int. J. Agric. Biol 12: 474–476.
Bryskier, A. 2005. Penicillins. In Antibacterial Agents. Antibacerial and Antifungal. Ed. A. Bryskier. ASM Press. Washington, DC. Pp. : 113-162.
Chandrashekar, M.A., K. Soumya Pai, N.S. Raju. 2014. Fungal Diversity of Rhizosphere Soils in Different
Agricultural Fields of Nanjangud
Taluk of Mysore District, Karnataka, India. Int.J.Curr.Microbiol.App.Sci
3(5): 559-566.
Dawar, S., S. Hayat, M. Anis and M.J. Zaki. 2008. Effect of Seed Coating Material In The Effi cacy of Microbail Aantagonists For The Control of Root Rot Fungi On Okra And Sunfl ower. Pak. J. Bot 40(3): 1269-1278.
Dawar, S., S. Hayat, M. Anis and M.J. Zaki. 2008. Effect of Seed Coating Material In The Effi cacy of Microbail Aantagonists For The Control of Root Rot Fungi On Okra And Sunfl ower. Pak. J. Bot., 40(3): 1269-1278.
Diba, K., P. Kordbacheh, S.H. Mirhendi, S. Rezaie, and M. Mahmoudi. 2007. Identification od Aspergillus species using morphological characteristics. Pak J Med Sci 23(6): 867-872.
Dolar, F.S. 2001. Antagonistic effect of Aspergillus melleus Yukawa on soilborne pathogens of Chickpea. Tarim Bilimleri Dergisi 8(2) : 167170.
Fakhrunnisa, M.H. Hasmi and A. Ghaffar. 2006. In vitro interaction of Fusarium spp. with other fungi. Pak. J. Bot 38(4): 1317-1322.
Gautam, A.K., and R. Bhadauria. 2012. Characterization of Aspergillus species associated with commercially stored triphala powder. African Journal of Biotechnology 11 (104): 16814-16823.
Haggag, W.M., and H. A.L. A. Muhamed, 2007. Biotechnological Aspects of Microorganisms Used in Plant Biological Control. American-Eurasian Journal of Sustainable Agriculture 1(1): 7-12.
Harman, G.E., C. R. Howell, A. Viterbo, I. Chet and M. Lorito. 2004. Trichoderma species – opportubistic, avirulent plant symbionts. Natural Reviews. Microbiology 2: 43 – 56.
Howell, C.R. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases : the history and evolution of current concepts. Plant Disease 87(1) : 4-9.
Idris, A.S., S. Nurahida and S. Shamala. 2008. In Vitro Methods for
Evaluation of Antagonistic Fungi Against Pathogenic Genoderma. MPOB Information Press 53: 1-2.
Indrawati. G., R.A. Samson, K. Van den Tweel-Vermeulen, A. Oetari dan I. Santoso. 1999. Pengenalan Kapang Tropik Umum. Yayasan Obor Indonesia. Universitas Indonesia (University of Indonsia Culture Collection) Depok, Indonsia dan Centraalbureau voor
Schirmmelcultures, Baarn, The Netherlands.
Jayalal, R.G.U. and N.K.B. Adikaram. 2007. Influence of Trichoderma harzianum metabolites on the development of green mould disease in the oyster mushroom. Cey.J.Sci. (Bio.Sci.) 36(1): 53-60.
Jha, B.K., S. Dey, M.D. Tamang, M.E. Joshy, P.G. Shivananda, K.N. Brahmandatan. 2006.
Characterization of candida species isolated from cases of respiratory tract infection. Kathmandu University Medical Journal 4(3): 290-294.
Katatny, M.H.E., W. Somitsch, K.-H. Robra, M. S. El-Katatny and G. M. Gübitz. 2000. Production of chitinase and β-1,3-glucanase by Trichoderma harzianum for control of the phytopathogenic fungus Sclerotium rolfsii. Food technol. biotechnol 38 (3) 173–180.
Lo, C.T. 1998. General mechanisms of action of microbial biocontrol agents. Plant Pathology Bulletin 7:155-166.
Mojica-Marin, V., H. A. Luna-Olvera, C. Fco, Sandoval-Coronado, B.Pereyra-Alférez, H. Lilia, Morales-Ramos, E. Carlos, Hernández-Luna and G. O. Alvarado-Gomez. 2008. Antagonistic activity of selected strains of Bacillus thuringiensis against Rhizoctonia solani of chili pepper. African Journal of Biotechnology 7 (9) :
1271-1276.
Naqvi, S.M.A., and S.K. Chauhan. 1980. Effect of root exudates on the spore germination of rhizosphere and
rhizoplane mycoflora of chilli (Capsicum annuum L.) cultivars. Plant and Soil 55: 397-402.
Pitt, J.I. and A.D. Hocking. 1997. Fungi and Food Spoilage. Blackie Avademic and Professional. Second Edition. London-Weinhein-New York-Tokyo-Melboune-Madras.
Rahman A., M.F. Begum, M. Rahman, M.A. bari, G.N.M. Ilias, and M.F. Alam. 2011. Isolation and identification of Trichoderma species from different gabitats and their use for bioconvertion of solid waste. Turk J Biol 35: 183-194.
Roeswitawati, D. 2007. Use of antagonist inoculums (fungus and bacteria) to Menekan suppress Lanas disease caused by Phytophthora parasitica var. nicotianae in tobacco. Journal of Agriculture science of Indonesia. Special Edition 3 : 418 – 426.
Samson, R.A., E.S. Hoekstra, and C. A.N. Van Oorschot. 1981. Introduction to Food-Borne Fungi. Centraalbureau Voor-Schimmelcultures. Institute of The Royal Netherlands. Academic of Arts and Sciences.
Samson, R.A., J. Varga, and J.C. Friscad. 2011. Taxonomic studies on the genus Aspergillus. Studies in Mycology 69: 1-103.
Siddiqui, I.A., S.S. Shaukat and A. Khan. 2004. Differential impact of some Aspergillus species on Meloidogyne javanica biocontrol by Pseudomonas fl uorescens strain CHA0. Applied Microbiology 39: 74-83.
Stanghellini, M.R., D.H. Kim, S.L. Rasmussen and P.A. Rorabaugh. 1996. Control of root rot pf peppers caused by Phytophthora capsici with a nonionic surfactant. Plant. Dis 80: 1113-1116.
Sudarma, I M. and D.N. Suprapta. 2011. Diversity of soil microorganism s in banana habitats with and without Fusarium wilt Symptom. J. ISSAAS 17(1) 147-159.
Sudarma, I M. and N. M. Puspawati. 2013. The epidemiology of wilt disease in pepper (Capsicum frutescens L.) at Banjarangkan, Klungkung. Research report (unpublished).
Sudbery, P., N. Gow, and J. Berman. 2004. The distinct morphogenic states of Candida albicans. Trends in Mircobiology. Review P: 1-8.
Tsafrir, J. 2015. Candida albicans and Mental and Mood Disorders. Boston Holistic Psychiatrist.
Vinale, F. , K. Sivasithamparam, E. L. Ghisalberti, R. Marra, S. L. Woo, M. Lorito. 2008. Trichoderma–plant– pathogen interactions. Review Article. Soil Biology & Biochemistry 40: 1–10.
Whipps, J.M. 2001. Microbial interactions and biocontrol in the rhizosphere. J.Exp.Bot 51(1): 487-511.
Wongpla, A., K. Lomthaisong. 2010. Changes in the 2DE protein profiles of chilli pepper (Capsicum annuum) leaves in response to Fusarium oxysporum infection. ScienceAsia 36: 259–2
asia oceania biosciences and biotechnology consortium • 92
Discussion and feedback