IDENTIFICATION OF ARBUSCULAR MYCORRHIZAL FUNGI IN RHIZOSPHERE SOIL OF SEVERAL GRASS SPECIES AND CACAO (Theobroma cacao L.) BASED ON ITS SPORE MORPHOLOGICAL CHARACTERISTICS
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • VOL. II NO. 1 • SEPTEMBER 2014
ISSN: 9 772303 337008
IDENTIFICATION OF ARBUSCULAR MYCORRHIZAL FUNGI IN RHIZOSPHERE SOIL OF SEVERAL GRASS SPECIES AND CACAO (Theobroma cacao L.) BASED ON ITS SPORE MORPHOLOGICAL CHARACTERISTICS
Ni Kadek Sintya Dewi, I Gede Ketut Susrama, Made Sritamin, Made Adnyana and I Gede Putu Wirawan
Department of Agroecotechnology, Faculty of Agriculture, Udayana University Jl. PB Sudirman, Denpasar 80232, Bali, Indonesia.
E--‐‑mail: igpwirawan@yahoo.com *Corresponding author
ABSTRACT
Arbuscular mycorrhizal fungi live in a symbiotic mutualism with plant roots and helps plant absorb nutrients and also able to live in various places. The study aimed to identify arbuscular mycorrhizal fungi in rizhosphere of several grass and cacao through microscopic method based on morphological characteristics and was conducted from December 2013 to March 2014. The methods used in this study were spore isolation using wet sieving and root staining techniques. The results showed that arbuscular mycorrhizal fungi spores found in Imperata cylindrica L. were spores of Glomus (Glomales: Glomeaceae with its vesicular and hyphae structure. The presence of spores and structures in Paspalum notatum were Acaulospora (Glomales: Acaulosporaceae and Gigaspora with its arbuscules, vesicules and hyphae structure. Spores and structures found in the Pennisetum purpureum were belong to genus of Glomus with hyphae and arbuscular structure. Spores and structures of mycorrhizal fungi in Cyperus rotundus are spores of the genus of Gigaspora (Glomales: Gigasporineae and spores of Glomus with internal hyphae structure. While spores and mycorrhizal structures in Cacao were found two types of spore belong to genus of Glomus with hyphae and vesicular structures.
Keywords: Glomus, Gigaspora, Acaulospora, Arbuscular Mycorrhiza Structure
INTRODUCTION
Production and productivity of crops in last 10 years, significantly declines were primarily due to ecological degradation of soil fertility and significantly increase of fertilizer price (Nasaruddin, 2012 . Efforts to overcome limitation of fertilizer and environmental damage are through application of soil biotechnology such as soil microbe services and technologies of natural fertilizer. One of the biotechnological developments in soil microbial fertilizer is by utilizing the symbiotic microbe such as arbuscular mycorrhizal fungi. Arbuscular mycorrhizal fungi is a fungus that lives in a symbiotic mutualism manner with plant roots and grow in the root cortex cells (Bundrett et al., 1996 .
Almost 100 percent Arbuscular mycorrhizal fungi observed in grasses of Gramineae family and 70% found in the rhizosphere of plants such
of cacao (Nasaruddin, 2009 . Gramineae grasses that can grow in a critical region allows better mycorrhizal fungi growth. The grasses mention above namely Bahia grass, Elephant grass, and Nut--‐‑grass. While the number of mycorrhizal fungi in cacao currently found lower than in grasses, because cacao is commonly a cultivated crop that maintained soil fertility resulted in lowering mycorrhizal fungi diversity. Khan (1993 mentions that mycorrhizal fungi can protect plant from certain toxic elements such as heavy metals and according to Wright and Uphadhyaya (1998 , arbuscular mycorrhizal fungi producing glycoprotein glomalin that is very correlated with soil aggregate stability. This study aims to identify arbuscular mycorrhizal fungi in rhizosphere of grasses and cacao and also to determine whether or not arbuscular mycorrhizal fungi infection in root tissues of grasses and in cacao.
MATERIALS AND METHODS
The research was conducted from December 2013 to March 2014. Rhizophere soil sampling and grass roots done in Penelokan village, while soil sampling and roots of cacao taken in Kintamani village and identification of arbuscular mycorrhizal fungi conducted in the Laboratory of Genetic Resources and Molecular Biology, Udayana University.
Materials needed in this study were 100 grams of rhizophere soil and root samples from the Cacao Cacao (Theobroma cacao L. and 4 species of grasses namely Bahia grass (Paspalum notatum , Reed grass (Imperata cylindrica , Elephant grass (Pennisetum purpureum , and Nut--‐‑grass (Cyperus rotundus , hydrogen peroxide, lactic acid 90%, glycerol, potassium hydroxide phosphate, and sterile water.
Necessary tools in this study were the autoclave, sieve size of 1 mm, 500 µμm, 212 µμm, 106 µμm, 53 µμm, tweezers, petridish, shaker, electric scales, erlenmeyer, micro tube, compound microscope and a stereo microscope, a water bath, slide glass and cover glass, and pasteur pipette.
PROCEDURES
Sampling of soil and roots were taken at each of the selected plants at 3 points in a depth of 30 cm from the base of the stems of plants, 100 grams of sample was weighed, and collected in a paper bag with its root sample, labeled and stored in a refrigerators.
Isolation of spores from soil samples, performed with wet filter technique. A total of 100 grams of dried soil samples were given water up to volume 1000 ml, stired for 2 minutes and filtered with a 1 mm sieve, 500 µμm, 212 µμm, 106 µμm and 53 µμm. The precipitate remaining in the sieve of 500 µμm, 212 µμm, 106 µμm and 53 µμm were sprayed with tap water and poured into centrifuge tubes. The precipitate was centrifuged at 5000 rpm for 5 minutes. The supernatant was then poured into a petri dish and then examined
under a stereo microscope for spore counting and making preparations for the identification of spores. Spores were theb observed under compound microscope and documented with a camera and spores identification was conducted based on spore photos and explanation that available in www.INVAM.wvu.edu.com website (INVAM, 2005 ..
Observation of the structure of arbuscular mycorrhizal fungi colonization of plant roots were conducted through root staining technique. Root segment was cut in a length of 5 cm, and then washed with water. Root sample was introduced into a solution of 10% KOH and heated at 250˚C for 10 minutes in a microwave oven and cooled at room temperature for 24 hours so that the roots will be white in color. KOH solution was then discarded and root samples were washed in running water for 10 minutes. Furthermore, the root samples were stored in a solution of 1% HCl and allowed to stand for one night and then HCl solution discarded. Root samples were then stored in a solution of 0.05% Trypan blue solution and replaced with lacto glycerol for color reduction process (destaining for one day. Then, the root pieces are covered with a cover glass and observed under compound microscope with magnification of 100 times.
Variables observed and analyzed in this study are spore morphological characters include shape, color and substanding hyphae. Genus determination was based on the morphological characteristics of each spore. Analysis of arbuscular mycorrhizal fungi infection seen through mycorrhizal structures (arbuscule, vesicule, and hyphae in roots after staining.
RESULTS AND DISCUSSION
Characteristics and Structure of Arbuscular Mycorrhizal Spores in Imperata cylindrical
The presence of spores in a Imperata cylindrica were belong to glomus with two types of characteristics. By staining with trypan blue solution mycorrhizal structures can be clearly visible where mycorrhizal fungi in the roots of
grass root sample have a vesicular structure and internal hyphae. The characteristics of each of arbuscular mycorrhizal spores and structures can be seen in Figure 1.
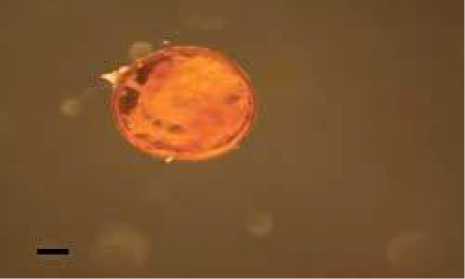
A

B
Fig. 1. Spores of Glomus and arbuscular mycorrhizal structures in Imperata cylindrica. (A Glomus type 1, (B The structure of arbuscular mycorrhizal fungi (a. Vesicular, b. Hyphae . (Magnification 100 times
Glomus spore type 2 has a characteristic brownish yellow, spherical shaped, its wall was clearly visible, smooth surface, did not have any ornaments and spore size was 125.39 µμm x 125.39 µμm.
Vesicular structures at the root has a spherical shape and react to the solution of trypan blue, while the internal hyphae shaped like fibers and absorb the color of the solution of trypan blue. Arbuscular structure couldn’t be found in the roots of grass might be because arbuscule that formed in Glomus too small to be
observed. Arbuscule life revolves around 4--‐‑6 days, then absorbed by its host plant (Brundrettet al., 1996 . Production of exudate on young reed is relatively higher resulted in higher mycorrhizal fungi population (Delvian, 1993 .
Characteristics and Structure of Arbuscular Mycorrhizal Spores in Bahia Grass
Presence of spores in a Bahia grass were belong to genus Acaulospora (Glomales: Acaulosporaceae . The results of staining with trypan blue on Bahia grass roots, showed that there was a complete structure of arbuscular mycorrhizal fungi which consist of arbuscule, vesicule, and internal hyphae . The characteristics of arbuscular mycorrhizal spores and structures can be seen in Figure 2.
A
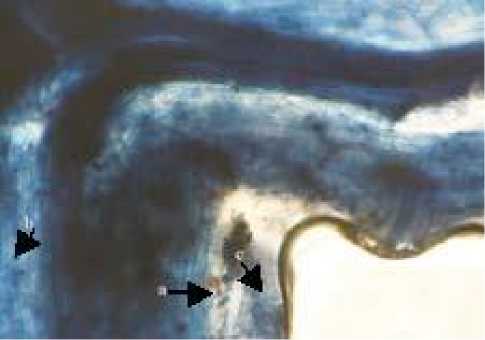
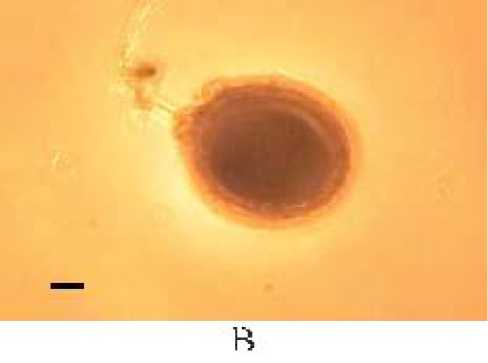
Fig. 2. Spores and arbuscular mycorrhizal structures in Bahia Grass. (A Acaulospora, (B The structure of arbuscular mycorrhizal (a. Arbuscular, b. Vesicular, c. Hyphae Internal (magnification 100 times
Characteristics of Acaulospora spores were solid maroon in color, has a spherical shape, its wall is clearly visible, hyphae have a stalk and spore size 413.63 µμm x 413.63 µμm. Observation result of mycorrhizal colonization in Bahia grass roots shown in Figure 2, mycorrhizal structures such as arbuscule, vesicule and internal hyphae clearly visible through the stainned root with trypan blue solution. In this study, beside Acaulospora, genus Gigaspora was also found in Bahia grass.
Characteristics and Structure of Arbuscular Mycorrhizal Spores in Elephant Grass
The results showed that the presence of spores in Elephant grass were genus Glomus spores with type one characteristics. The characteristics and structure of those mycorrhizal spores can be seen in Figure 3.
B
Fig. 3. Spores of the genus Glomus and arbuscular mycorrhizal structures in Elephant Grass. (A Glomus type 2, (B The structure of arbuscular mycorrhizal (a.
Vesicular, b. Arbuscular . (Magnification 100 times
Glomus spores of type 2 characteristic are brown spores, spherical shape, thick spore wall, having the trappings of sand in the middle of the cell wall and spore measuring 341.09 µμm x 341.09 µμm.
Observations on mycorrhizal structures using compound microscope at magnification of 100 times were seen arbuscular and vesicular structures in the Elephant grass. The existence of the primary structure shows that mycorrhizal colonization in root grass occured. Other major structures such as the internal hyphae not seen in this observation. According to Bundrett et al., (1996 , the internal hyphae might had had developed into a vesicule and arbuscule.
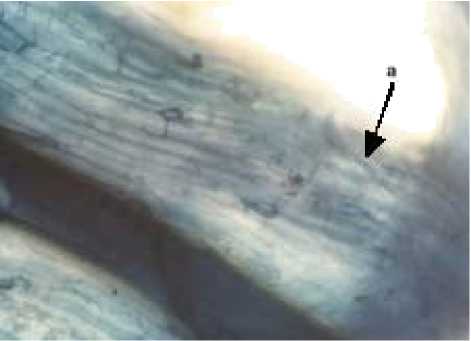
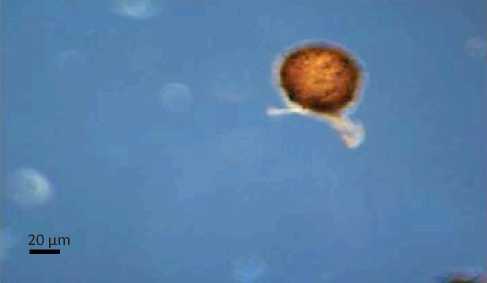
Characteristics and Structure of Arbuscular Mycorrhizal Spores in Cyperus rotundus
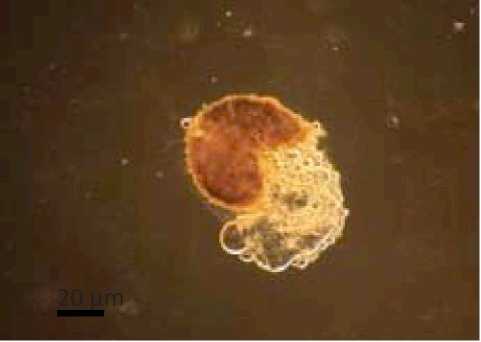
The results showed that the presence of spores in Cyperus rotundus with one types of characteristics of the spores and spore types Gigaspora (Glomales: Gigasporineae . Characteristics of each spore and structure of arbuscular mycorrhizal hyphae can be seen in Figure 4. A
Characteristics of Gigaspora spores were brown in color, has a spherical shape, spore wall is clearly visible, had bulbous suspensor, and spores measuring 146.48 µμm x 146.48 µμm. Glomus type 3 was brown, had a spherical shape, clearly visible spore walls, had a wall surface pattern like orange peel and spore size 312.80 µμm x 312.80 µμm.
Observations indicate the structure of mycorrhizal hyphae in Cyperus rotundus had internal structure. The growth of external hyphae will be occured if the internal hyphae grow from cortex through epidermis. According to Widyastuti et al. (2005 mention that external hyphae function is to support reproductive function as well as for transporting carbon and other nutrients into spores, in addition it is also to absorb nutrients from the soil for use by plants. Environmental factors such as soil type and altitude influencing the formation of spore type (Dewi, 2007 .
A
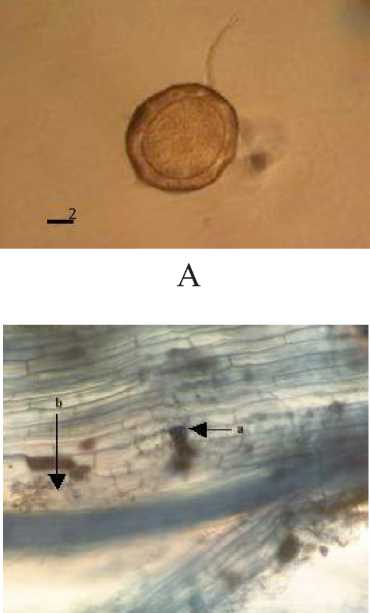
Characteristics and Structure of Arbuscular Mycorrhizal Spores in Cacao
Spores found in a cacao plant belong to genus of Glomus. Observation on the structure of mycorrhizal in roots of cacao showed vesicular structures and hyphae. Characteristics of one of mycorrhizal spore and structure can be seen in Figure 5.
A

B
C
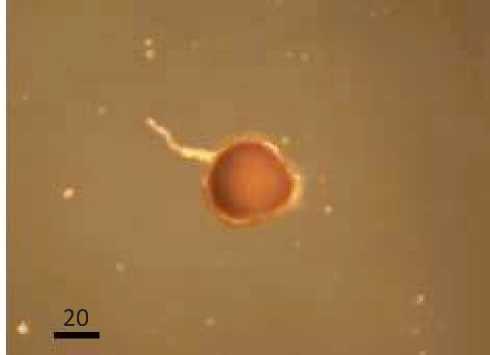
Fig. 4. Arbuscular mycorrhizal spores and structure of the Cyperus rotundus . (A Gigaspora (B Glomus types 3, (C The structure of arbuscular mycorrhizal fungi (a. hyphae (Magnification 100 times .
B
Fig. 5. Spores of the genus Glomus and arbuscular mycorrhizal structures in Cacao. (A Glomus type 4, (B The structure of arbuscular mycorrhizal fungi (a. hyphae, b. Vesicule . (magnification 100 times .
Morphological characteristics of Glomus spore type 4 is dark brown in color, has a spherical shape, clearly visible spore walls, patterned like an orange peel, and spores measuring 123.15 µμm x 123.15 µμm. The observation of mycorrhizal structures with a compound microscope magnification of 100 times
indicated the presence of vesicular structures and internal hyphae on the roots of cacao. Vesicular structures at the root has a spherical shape and react positively to the solution of trypan blue, while the internal
hyphae shaped like fibers and absorb the color of the solution of trypan blue.
Spores found in Imperata cylindrica was a genus of Glomus (Glomales: Glomeaceae with one type of spore and spores in a Bahia grass was genus Acaulospora (Glomales: Acaulosporaceae . One type of spore found in the Elephant grass that belong to Glomus. Spores which were found in Cyperus rotundus were Gigaspora spores and Glomus spores. While the presence of spores in cacao were genus Glomus with one type of spore.
Roots of Imperata cylindrica and cacao having vesicular structure and mycorrhizal hyphae, whereas arbuscular structures found in Elephant grass roots. Colonization of the complete structure (arbuscule, vesicule, and internal hyphae founds in Bahia grass roots, while at the grassroots of Cyperus rotundus only internal hyphae was visible.
Type and the number of spore in the overall sample is fairly low, therefore it is necessary for efforts to increase the population by trapping spores and spore propagation so more spores available for more accurate identification. Molecular identification should be perfomed and more data needed to get a better understanding of the complexity of mycorrhizal fungi.
REFERENCES
Brundrett M, Bougher N, Dell B, Grove T and
Malajczuk N. 1996. Working with Mycorrhizas in Forestry and Agriculture. ACIAR Monograph 32.
Nasaruddin. 2009. Cacao Cultivation and Its Some
Physiological Aspects (In Indonesian
Language . Indonesian Forest Foundation and Cocoa research Group (CRG , Faculty of Agriculture, Hasanuddin University. Makassar. 164p.
Widyastuti H, Guhardja E, Soekarno N, Darusman LK, Goenadi DH and Smith S. 2005. Using arbuscular mychorrhizal fungi spore as innoculum to increase growth and nutrient absorbtion in Oil Palm seedling (In Indonesian Language . Journal of “Menara Perkebunan”, p. 26--‐‑34.
Delvian. 2003. Diversity and Utilization Potential of Arbuscular Mycorrhizal Fungi (AMF at Forest Beach (In Indonesian Language . Graduate Program Desertation. Bogor Agricultural University. Bogor.
Dewi RI. 2007. Role, Prospects and Constraints in Using Endomycorrhizal Fungi (In Indonesian Language . Faculty of Agriculture, Padjadjaran University. Bandung.
INVAM. 2005. International Culture Collection of (Vesicular Arbuscular Mycorrhizal Fungi. Acauslospora foveata (reference accession BR861 . http: Ninvarn.caf.wvu.
edu/iungi~tlxonorny/Aca~losporaceae/ Acaulospora/foveata/foveata.html (July 7, 2005 . Accessed October 1, 2013.
Khan AG. 1993. Effect of various soil environment stresses on the occurance, distribution and effectiveness of VA mycorrhizae. Biotropia 8: 39--‐‑44.
Wright SF and Upadhyaya A. 1998. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant and Soil 198: 97 – 107.
asia oceania biosciences and biotechnology consortium • 31
Discussion and feedback