Phytochemical Analysis of Bulung Boni (Caulerpa cylindracea S.) N- Hexane Extract with Gc-Ms Method and Toxicity Test on Mice (Mus musculus L.)
on
Phytochemical Analysis of Bulung Boni (Caulerpa cylindracea S.) N- Hexane Extract with Gc-Ms Method and Toxicity Test
on Mice (Mus musculus L.)
Silalahi, M.D.B., Wirawan, I.G.P., Wijaya, I.N., Suada, I.K., Phabiola T.A. & Astaykinta, A.
PHYTOCHEMICAL ANALYSIS OF BULUNG BONI (Caulerpa cylindracea S.) N-HEXANE EXTRACT WITH GC-MS METHOD AND TOXICITY TEST ON MICE (Mus musculus L.)
May Disa Br Silalahi1, I Gede Putu Wirawan1*, I Nyoman Wijaya1, I Ketut Suada1, Trisna Agung Phabiola1, and Angelika Astaykina2
-
1Faculty of Agriculture, Udayana University, Jl. PB. Sudirman, Denpasar, Bali 80232, Indonesia
-
2Department of Soil Science, Lomonosov Moscow State University, 119234, Leninskie Gory, 1, Russia
*Corresponding author: igpwirawan@unud.ac.id
ABSTRACT
Received:
12 December 2022
Accepted:
20 July 2022
Published:
3 August 2023
One of the marine resource commodities owned by Indonesia is seaweed. Bulung boni is one type of seaweed that grown in Indonesia. Bulung boni is the regional name of Caulerpa cylindracea. Bulung boni is usually used as a food and medicine for most people in Bali. The purpose of this research is to study the content of phytochemical compounds in n-hexane extract of bulung boni and determine the toxicity effect on mice (Mus musculus). The methods used in this study were maceration, GC-MS (Gas Chromatography-Mass Spectrometry), observation, and calculation with the Thomson and Weil formula. This study used 24 mice as experimental animals. Mice were divided into 6 treatment groups, group 1 as control was given 1% Na-CMC solution, groups 2-6 were given test extracts with doses, 100, 200, 300, 400, 500 mg/30 g BW mice. The results showed the highest compound contained in the n-hexane extract of bulung boni was Hexadecanoic acid, methyl ester with an AUC value of 35.21%. Toxicity tests on mice showed no deaths experienced by mice, so the LD50 value determined was pseudo at 16.6 g/ kg BW and fell into the practically nontoxic category.
Keywords: Bulung boni, phytochemical, GC-MS, toxicity, LD50
INTRODUCTION
Bulung boni is one type of seaweed that grows in Indonesia. Bulung Boni is the regional name of Caulerpa cylindracea. This seaweed is usually used as a vegetable by the Balinese people. In the study of Julyasih (2009) stated the ethanol extract of
bulung boni contains 37,249,000 µg carotenoids in 100 mg samples.
Carotenoids are antioxidants that are very potential in protecting lipid membranes against peroxidation (Siems et al., 2002). The use of antioxidant compounds has recently grown rapidly
along with the increasing knowledge of free radical activity against several degenerative diseases (Hanani et al., 2005). Epidemiological studies shows that consuming antioxidants derived from natural materials such as vegetables and fruits shows protective effects against various degenerative diseases such as coronary heart disease, stroke, and cancer (Budiana, 2008). Therefore, bulung boni has the potential to be developed into processed food and medicine products.
In processing a raw material, the content of the compounds in the material must be known. Phytochemical analysis is a test conducted to identify bioactive components in a material. Bioactive components are active compounds in functional foods that are responsible for metabolic reactions that benefit health (Subroto, 2008). In identifying phytochemical compounds, the solvent used in extraction is very influential on the content of compounds that can be identified. This is due to the principle of like dissolves like, which is a solvent tends to dissolve compounds that have the same level of polarity. Carotenoids are a group of compounds with low polarity so that to get this group of compounds used solvents with low polarity such as n-hexane. Based on the research of Wahyuni and Widjanarko
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2023.v10.i02.p06
(2015), the highest carotenoid content was produced by n-hexane solvent. N-hexane is mostly used as an organic solvent that is inert due to its non-polarity. There are several techniques used to analyze phytochemical compounds, one of which is using the GC-MS (Gas ChromatographyMass Spectrometry) method.
Toxicity tests on a raw material are also very important and necessary before the material is processed into food or medicine. The existence of a toxicity test is carried out to determine the toxic effects of a compound and its capacity to affect an organism. Acute toxicity tests are part of preclinical tests designed to measure the toxic effects of a compound within 24 hours interval. The median lethal dose or LD50 is a statistical benchmark after a single dose administration that is often used to express the level of toxic dose as quantitative data. Clinical symptoms, physiological
symptoms and toxic mechanisms as qualitative data (Jenova, 2009).
Based on the description above, until now there has been no report on the level of safety in the use of bulung boni and what compounds are contained in the n-hexane extract of bulung boni. So it is important to conduct toxicity testing of n-hexane extract of bulung boni and its phytochemical analysis. This is due to the possible content
of bulung boni n-hexane extract is harmful to humans if consumed at doses that are not recommended and in long term use.
MATERIALS AND METHODS
This research was conducted from August 2022 to October 2022. The place of implementation of the preparation n-hexane extract bulung boni at the Laboratory of Genetic Resources and Molecular Biology, Udayana University. The place of phytochemical compound analysis of bulung boni was conducted at the Forensic
Laboratory of the Denpasar branch of the National Police Criminal Investigation Unit. The implementation of the bulung boni toxicity test on mice was carried at the Biotechnology Laboratory of the Faculty of Agriculture, Udayana University. The bulung boni samples used in this study were collected from Serangan Island, Denpasar, Bali and the test animals used in this study were taken from Mice Breeder, Denpasar, Bali. Figure 1 shows the maps of bulung boni site
Figure. 1. The Maps of Bulung Boni Site
The data generated from GC-MS analysis is processed in-silico by matching the compounds in the chromatogram with the compounds in the literature. The data collected in the toxicity test research on mice is primary data from the observation of experimental animals, both control group and treatment groups. The data obtained are qualitative data and quantitative data.
Qualitative data was obtained by observing the clinical symptoms experienced by the mice. Quantitative data was obtained by calculating the LD50, which is the number of mice that died and those that were still alive in each group. LD50 will be calculated using the Thomson and Weil method with the equation (1).
log m = log D + d(f+1) ………………………………………………………………….(1)
Description:
m = LD50 value
D = smallest dose used
d = log of the multiple of the dose
f = a value in Weil's table due to a certain mortality rate (r)
Preparation of Bulung Boni Extract
The bulung boni that has been obtained is washed thoroughly then dried in the air for 2 days and then dried using an oven for 5 days at a temperature below 45oC. Then ground using a blender and sifted with a 60 mesh sieve, then added n-hexane solvent and then evaporated using a rotary evaporator to get a condensed extract of bulung boni.
Determination of Phytochemical Compounds in Bulung Boni Extracts
The analysis was performed using Agilent 7890B MSD 5977B and Wakosil ODS/5C18-200 silica column with size 4.6 x 200 mm using N2 as a carrier. The injection temperature used was 290oC for 27 minutes with constant flow and a flow rate of 1 ml/Min. Identification was conducted by looking at and comparing the retention time on each chromatogram peak with the reference database.
Administration of Bulung Boni Extract to Mice
The experimental animals used were male mice aged 60 days. The mice used
were healthy as many as 24 mice with a weight 20-30 g. Mice were divided into 6 groups, namely group 1 as control and groups 2-6 as treatment groups. Previously, the mice were acclimatized first for 1 week to adapt the mice's living environment to the laboratory environment. Mice were also fed (can still be given a drink) for 14 hours before treatment. Each group of mice was put into 1 cage. Each group was treated as follows:
(K) given 1% Na-CMC
(P1) given 100 mg/30 g BW of extract
(P2) given 200 mg/30 g BW of extract
(P3) given 300 mg/30 g BW of extract (P4) given 400 mg/30 g BW of extract (P5) given 500 mg/30 g BW of extract.
The treatment was given orally (single dose) to mice using a sonde. The observation parameters in this study were the number of mortality mice after 24 hours of administration n-hexane extract bulung boni and toxicity symptoms experienced by mice for 14 days. Symptoms of toxicity observed were hypersalivation,
convulsions, diarrhea, behavioral changes, and tremor.
RESULTS AND DISCUSSION
Bulung Boni (Caulerpa cylindracea)
Bulung boni has a green thalus consisting of many erect branches that are about 2.5-6.0 cm tall. The main stem measures between 16-22 cm. There is a flatshaped sphere at the top of the branch, the
length of each branch top is about 2.5-10.0 cm (Trono and Ganzon, 1988). Bulung boni is mostly found in coastal areas that have coral reef flats, growing on dead substrates, dead coral fragments, mud sand and mud. Figure 2 shows morphology structures of bulung boni (Caulerpa cylindracea)

Figure. 2. Morphology Structures of Bulung Boni (Caulerpa cylindracea)
Phytochemical Analysis
The chromatogram of GC analysis resulted in chromatogram peaks showing the compounds contained in the n-hexane extract of bulung boni as shown in Figure 3. The n-hexane extract of bulung boni contained two compounds that had a high abundance, such as Hexadecanoic acid, methyl ester, with an AUC value of 35.21% and Heptadecane with an AUC value of 12.33%. From these data can be concluded that the n-hexane extract of bulung boni is
dominated by fatty acid organic compounds. Research by Abubacker (2013) stated that hexadecanoic acid, methyl ester has potential as an antifungal. Research by Kim et al., (2013) stated that heptadecane functions as an antioxidant compound that could block fatty acid synthesis and improve several diseases associated with oxidative stress. Heptadecane also has activity in suppressing breast and cervical cancer cell viability (Mishra et al., 2019). identified chemicals compounds in bulung boni chromatogram shown in Table 1.
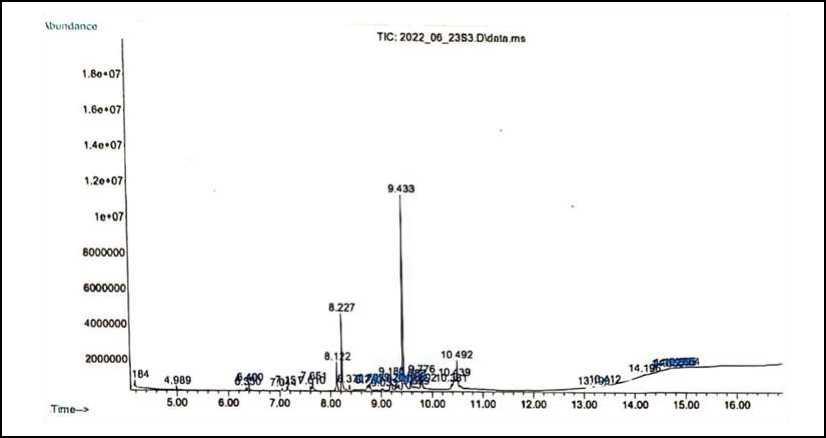
Figure 3. Chromatogram Peak N-hexane Extract of Bulung Boni (Caulerpa cylindracea)
Table 1. Identified Chemicals Compounds in Bulung Boni Chromatogram
|
Chemicals |
RT |
AUC |
Formula |
|
Phenol, 2 methoxy- |
4.184 |
1.21 |
C7H8O2 |
|
Tetradecane |
6.400 |
1.11 |
C14H30 |
|
2,4-Di-tert-butylphenol |
7.151 |
0.78 |
C14H22O |
|
Hexadecane |
7.651 |
1.19 |
C16H34 |
|
8-heptadecene |
8.122 |
5.32 |
C17H34 |
|
Heptadecane |
8.277 |
12.33 |
C17H36 |
|
Methyl tetradecanoate |
8.372 |
0.97 |
C15H30O2 |
|
Octadecane |
8.773 |
1.09 |
C18H38 |
|
z-5-nonadecene |
9.183 |
3.42 |
C19H38 |
|
e-14-hexadecenal |
9.183 |
3.42 |
C16H30O |
|
Nonadecane |
9.294 |
1.23 |
C19H40 |
|
Eicosane |
9.294 |
1.23 |
C20H42 |
|
Hexadecanoic acid, methyl |
9.433 |
35.21 |
C17H34O2 |
|
ester | |||
|
Hexadecanoic acid, ethyl |
9.776 |
3.00 |
C18H36O2 |
|
ester | |||
|
11,13-dimethyl-12-tetradecen-1-ol acetate |
10.492 |
6.42 |
C18H34O2 |
|
10-heneicosene (c,t) |
10.492 |
6.42 |
C21H42 |
RT, Retention Time (min.); AUC, Area Under the Curve (%)
Other compounds found in the n-hexane extract of bulung boni are Phenol, 2 methoxy- that functions as an antioxidant, flavoring agent, expectorant, and local
anesthetic (PubChem, 2005). Tetradecane that functions as a chemical intermediary and solvent (PubChem, 2004). 2,4-Di-tert-butylphenol that functions as an
antioxidant, and marine metabolite (PubChem, 2005). Hexadecane that functions as an essential oil component and non-polar solvent (PubChem, 2004). 8-heptadecene that functions as an anti-mite (Nazzi, 2002). Methyl tetradecanoate that functions as a flavoring ingredient and aroma agent (PubChem, 2005). Octadecane that functions as a solvent, as a calibration standard, and as an antimicrobial (Manikandan et al., 2017). Nonadecane that functions as an organic synthetic material,
fragrance material, and detergent material (PubChem, 2004). Eicosane that functions as an anti-hyperglycemia (Rodrigues et al., 2017). Hexadecanoic acid, ethyl ester that functions as a flavoring agent (PubChem, 2005).
Toxicity Test on Mice
The results of observations of clinical symptoms experienced by mice for 14 days can be seen in Table 2.
Table 2. Clinical Symptoms Experienced by Mice for 14 Days
|
Groups |
Symptomps | ||||
|
Hypersalivation |
Convulsion |
Diarrhea |
Behavior |
Tremor | |
|
Control |
Normal |
Normal |
Normal |
Normal |
Normal |
|
I |
Normal |
Normal |
Normal |
Normal |
Normal |
|
II |
Normal |
Normal |
Normal |
Normal |
Normal |
|
III |
Normal |
Normal |
Normal |
Normal |
Normal |
|
IV |
Normal |
Normal |
Normal |
Normal |
Normal |
|
V |
Normal |
Normal |
Normal |
Normal |
1 Tail |
Observation of hypersalivation toxicity symptoms was indicated by the continuous salivation of mice. Observation of hypersalivation toxicity symptoms in the treatment group did not show any symptoms, therefore salivation in mice in the treatment group and control group was normal. Symptoms of convulsion toxicity was indicated by uncontrolled muscle contractions on mice. In the observation of the symptoms convulsions also showed normal conditions in both the treatment and
control groups. Symptoms of diarrhea toxicity are indicated by the condition of liquid feces and repeatedly. Observation of diarrhea in this study also showed no symptoms in the treatment groups and control group. Changes in behavior and activity of mice in the treatment groups and control group also showed normal condition. Tremor toxicity symptoms were observed on 1 mice in the 500 mg/30 g BW dose treatment group. This symptom was indicated by the trembling of the mice's
body at the time of rest and activity. Mice with tremor occurred in the first 20 minutes after being given the test preparation. Tremor may be caused by a disturbance in the neuromuscular system of mice after administration of n-hexane extract of bulung boni at a dose of 500 mg/30 g BW. Neuromuscular occurs due to a failure of interaction between motor nerves and muscle cells which causes muscle contraction disorders (Lu and Kacew, 2002). Mice that experience tremors then return to normal the following day.
Determination of LD50
Determination of the LD50 value is based on the presence or absence of death in the test animals. The results of observations made during 14 days showed no deaths. The data that has been obtained from each group within 24 hours until day 14, then used to calculate the LD50 value using the Thomson and Weil formula. With no death of test animals, it shows that there is no f factor obtained from the Thomson and Weil table, so the calculation of the LD50 value cannot be continued. This is in accordance with the criteria for acute toxicity tests conducted to assess LD50 that based on the agreement taken by experts, if the maximum dose given does not cause the death of test animals, then the LD50 is expressed with pseudo LD50 or not the real
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2023.v10.i02.p06
LD50 (Loomis, 1978). According to Armansyah et al., (2016) in the acute toxicity test that has been carried out does not cause the death of test animals after giving a single dose of test substance, so to determine the LD50 value of the extract, the highest dose given to the test animals is used. Based on this statement, the apparent LD50 value of bulung boni n-hexane extract in this study is 16.6 g/ kg BW and bulung boni n-hexane extract is included in a practically non-toxic category. If up to a dose of 5000 mg/ kg BW does not cause death, then the test does not need to be continued using a higher dose of test substance (BPOM, 2014). In general, the smaller the LD50 value the more toxic the compound, and vice versa the greater the LD50 value the lower the toxicity.
CONCLUSION
The n-hexane extract of bulung boni (Caulerpa cylindracea) mostly contains fatty acid derivative compounds. The most abundant compound contained in the extract is the compound Hexadecanoic acid, methyl ester with an AUC value of 35.21%. The n-hexane extract of bulung boni (Caulerpa cylindracea) at a dose of 500 mg/30 g BW did not result in death in 50% of the test animals. The LD50 value determined was a pseudo of 16.6 g/ kg BW
and fell into the practically non-toxic category.
ACKNOWLEDGEMENT
This Study was supported by Udayana University Research Grant No.: B/78.331/ UN.14.4.A/PT.01.03/2022
REFERENCES
Abubacker, M. N., & Deepalakshmi, T. (2013). In vitro Antifungal Potentials of Bioactive Compound Methyl Ester of Hexadecanoic Acid Isolated from Annona muricata Linn. (Annonaceae) Leaves. Biosciences Biotechnology Research Asia Vol. 10(2), 879-884.
Armansyah, T. T. R., Sudi, I., Amalia, S., Rosmaidar, R., Nuzul, A., Budianto, P., Dwinna, A., Hamdan, H. (2016). Uji Toksisitas Akut Ekstrak Etanol Daun Malaka (Phyllantus emblica) Terhadap Mencit (Mus musculus). Banda Aceh: Fakultas Kedokteran Universitas Syiah Kuala.
BPOM. (2014). Pedoman Uji Toksisitas Nonklinik Secara In Vivo. Badan Pengawas Obat dan Makanan Republik Indonesia.
Budiana. (2008). Memahami Dampak Kolesterol. Available from: URL: http:// www.dewansfamilymultiply.com.
Hanani, E., Mun’im, A., & Sekarini, R.
(2005). Identifikasi Senyawa Antioksidan dalam Spons
Callyspongia sp. dari Kepulauan Seribu. Majalah Ilmiah
Kefarmasian, 3(2): 127-133.
Jenova, R. (2009). Uji Toksisitas Akut yang Diukur Dengan Penentuan LD50 Ekstrak Herba Putri Malu (Mimosa pudica l.) Terhadap Mencit balb/c. Laporan Akhir Penelitian Karya Tulis Ilmiah. Fakultas Kedokteran Universitas Diponegoro. Semarang.
Julyasih, K.S.M., Wirawan, I.G.P., Harijani, W.S., dan Widajati, W. (2009). Aktivitas Antioksidan beberapa Jenis Rumput Laut
(Seaweeds) Komersial di Bali.
Seminar Nasional ‘Akselerasi Pengembangan Teknologi Pertanian dalam Mendukung Revitalisasi Pertanian’. Fakultas Pertanian & Lppm UPN Veteran. Jawa Timur.
Kim, D. H., Min, H. P., Yeon, J. C., Ki, W.C., Chan, H. P., Eun, J. J., Hye, J.A., Byung, P. Y., & Hae, Y. C. (2013). Molecular Study of Dietary Heptadecane for the AntiInflammatory Modulation of NF-kB in the Aged Kidney . Department of Pharmacy, College of Pharmacy, Aging Tissue Bank, Pusan National
University, Gumjung-gu, Busan, Republic of Korea.
Loomis, T. A., Lea., and Febiger. (1987). Essential of Toxicology. Journal of Pharmaceutical Sciences. 3rd ed. 600 Washington Square.
Philadelpia.
Lu, F. C., & Kacew, S. (2002). ‘Coventional Toxicity Studies’ in Lu, F.C. and Kacew, S., Basic Toxicology: Fundamentals, Target Organs, and Risk Assessment, 4th edition, Taylor and Francis, London and New York, pp 77.
Manikandan, G., Vimala R. A., Divya, C and Ramasubbu, R. (2017). GC-MS Analysis Of Phytochemical Constituents In The Petroleum Ether Leaf Extracts Of Millettia peguensis,” vol. 8, no. 9, pp. 144– 150.
Mishra, S., Verma, S. S., Rai, V., Awasthee, N., Arya, J. S., Maiti, K. K., & Gupta, S. C. (2019). Curcuma raktakanda Induces Apoptosis and Suppresses Migration in Cancer Cells: Role of Reactive Oxygen Species. Biomolecules 9(4): 159.
Nazzi, F., Norberto, M., Giorgio, D. V. (2002). (Z)-8-Heptadecene from Infested Cells Reduces the Reproduction of Varroa destructor Under Laboratory Conditions.
Journal of Chemical Ecology, Vol. 28, No. 11. Italy.
PubChem. (2004). Hexadecane.
https://pubchem.ncbi.nlm.nih.gov/c ompound/ 11006
PubChem. (2004). Nonadecane.
https://pubchem.ncbi.nlm.nih.gov/c ompound/ 12401
PubChem. (2004). Tetradecane.
https://pubchem.ncbi.nlm.nih.gov/c ompound/ 12389
PubChem. (2005). 2,4-Di-tert-butylphenol. https://pubchem.ncbi.nlm.nih.gov/ compound/7311
PubChem. (2005). Hexadecanoic acid, ethyl ester.
https://pubchem.ncbi.nlm. nih.gov/compound/12366
PubChem. (2005). Methyl tetradecanoate. https://pubchem.ncbi.nlm.nih.gov/ compound/31284
PubChem. (2005). Phenol, 2 methoxy-. https://pubchem.ncbi.nlm.nih.gov/ compound/460
Rodrigues, M. J., Custódio, L., Lopes, A., Oliveira, M., Neng, N. R., Nogueira, J. M., Martins, A., Rauter, A. P., Varela, P., & Barreira, L. (2017). Unlocking the in vitro antiinflammatory and antidiabetic
potential of Polygonum maritimum. Pharmaceutical biology 55(1): 1348-1357.
Siems, W., Quast, S., Carluccio, F.,
Wiswedel, I., Hirsch, D., Augustin, W., Hampi, H., Riehle, M.,
Sommerburg, O. (2002). Oxidative Stress in Chronic Renal Failure as A Cardiovascular Risk Factor. Clin. Nephrol. 58 (Suppl 1), S12–S19 (Jul).
Subroto, M. A. (2008). Real Food, True Health. Makanan Sehat Untuk Hidup Lebih Sehat. PT AgroMedia Pustaka, Jakarta.
Trono, J. R. G . C., & Ganzon, E. T. (1988). Philippine Seaweed. Publ. by National Book Store. Inc : 327 pp.
Wahyuni, D. T., & Widjanarko, S. B. (2015). Pengaruh Jenis Pelarut dan Lama Ekstraksi terhadap Ekstrak Karotenoid Labu Kuning dengan Metode Gelombang Ultrasonik. Jurnal Pangan dan Agroindustri Vol. 3 No 2 P.390-401.
51
Discussion and feedback