EFFECTS OF HIGH-FAT DIET FEEDING ON LIPID PROFILE IN RATS (Rattus norvegicus)
on


ISSN: 2597-8012 JURNAL MEDIKA UDAYANA, VOL. 11 NO.10,OKTOBER, 2022
Iλ Idirectoryof OPEN ACCESS
I_J <JΛAU JOURNALS
Diterima: 2022-04-25 Revisi: 2022-08-28 Accepted: 25-09-2022
EFFECTS OF HIGH-FAT DIET FEEDING ON LIPID PROFILE IN RATS (Rattus norvegicus)
Putu Teja Prawitha Pinatih1, Ni Nyoman Ayu Dewi2, I Wayan Gede Sutadarma2 1Program Studi Sarjana Kedokteran dan Profesi Dokter, Fakultas Kedokteran Universitas Udayana 2Departemen Biokimia, Fakultas Kedokteran Universitas Udayana
E-mail: tejaprawitha@gmail.com
ABSTRAK
Penyakit kardiovaskular merupakan pembunuh utama di dunia. Dasar penyakit kardiovaskular adalah proses dislipidemia. Sehingga, diperlukan model dislipidemia untuk penelitian tentang terapi penyakit kardiovaskular. Tujuan penelitian ini adalah untuk meneliti efek dari pemberian diet tinggi lemak terhadap profil lipid tikus sebagai model dislipidemia. Pakan standar untuk tikus dicampurkan dengan 30% lemak babi dan 20% kuning telur untuk menghasilkan pakan tinggi lemak. Enam ekor tikus Wistar berumur tiga bulan dengan berat 200±10g dibagi menjadi dua kelompok perlakuan, 3 ekor diberi makan pakan standar (NF) dan 3 ekor diberi makan diet tinggi lemak (FF). Tikus diberi makan selama 6 minggu sesuai kelompok perlakuan. Sampel darah kemudian dianalisis untuk mengukur kadar kolesterol dengan metode CHOD-PAP, kadar trigliserida dengan metode GPO-PAP, dan kadar HDL dengan metode homogenous enzyme colorimetric assay. Kadar LDL dihitung menggunakan rumus Friedewald. Hasil analisis sebagai berikut. Kolesterol total: 55.00 mg/dL pada kelompok NF; 92.67 mg/dL pada kelompok FF (p=0.027). Trigliserida: 78.33 mg/dL pada kelompok NF; 171.67 mg/dL pada kelompok FF (p=0.014). HDL: 32.00 mg/dL pada kelompok NF; 43.00 mg/dL pada kelompok FF (p=0.422). LDL: 7.33 mg/dL pada kelompok NF; 15.33 mg/dL pada kelompok FF (p=0.437). Hasil penelitian ini menunjukkan peningkatan signifikan pada kolesterol total dan trigliserida namun tidak ada perbedaan bermakna pada kolesterol HDL dan LDL; komposisi diet pada penelitian ini belum sesuai untuk studi dislipidemia yang diarahkan sebagai model aterosklerosis.
Kata Kunci: diet tinggi lemak., dyslipidemia., profil lipid
ABSTRACT
Cardiovascular diseases are the world’s leading cause of death. The pathological basis of most cardiovascular diseases is dyslipidemia. Thus, animal model of dyslipidemia should be designed. The aim of the present study was to research the effects of feeding high-fat diet on lipid profile in rats as a model of dyslipidemia. Standard rat feed was mixed with 30% pig fat and 20% egg yolks to produce the high-fat diet. Six three-months old Wistar rats weighing approximately 200±10 g was divided into two groups of three rats each: normal-fed (NF) and high-fat diet fed (FF). The rats were fed for 6 weeks as follows: standard lab rat diet for NF group; and high-fat diet for FF group. Blood sample was then collected and analyzed for serum total cholesterol by CHOP-PAP method, triglycerides by GPO-PAP method, and HDL cholesterol by homogeneous enzymatic colorimetric assay. LDL cholesterol was mesured using Friedewald’s formula. The results were as follows. Total cholesterol: 55.00 mg/dL in NF group; 92.67 mg/dL in FF group (p=0.027). Triglycerides: 78.33 mg/dL in NF group; 171.67 mg/dL in FF group (p=0.014). HDL cholesterol: 32.00 mg/dL in NF group; 43.00 mg/dL in FF group (p=0.422). LDL cholesterol: 7.33 mg/dL in NF group; 15.33 mg/dL in FF group (p=0.437). The result found a significant increase of total cholesterol and triglycerides between the groups but no difference of HDL and LDL; suggesting the current dietary regimen is not appropriate for further studies on dyslipidemia, particularly as model for atherosclerosis.
Keywords: high-fat diet., dyslipidemia., lipid profile
INTRODUCTION
According to data from the World Health Organization (WHO), cardiovascular diseases are the world’s leading cause of death. During the year 2015, it is estimated that 17,7 million deaths worldwide are due to cardiovascular diseases, making up 31% of all deaths worldwide. From those numbers, 7,4 million deaths are caused by coronary heart disease (CHD), and 6,7 million deaths are caused by stroke. More than three-fourth of fatal cardiovascular events occurs in low- to middle-income countries, such as Indonesia.1
The Riset Kesehatan Dasar (Riskesdas) by the Indonesian Ministry of Health stated that in 2013, the prevalence of CHD in Indonesia is 0,5% of the total population, or approximately 883.447 people. The Sample Registration System (SRS) Survey in Indonesia at 2014 found that CHD accounts for 12,9% of all deaths in Indonesian, making it the leading cause of death after stroke. Cardiovascular diseases have also become a burden for Indonesia’s national funds, with its cost of treatment increasing each year. As stated by the Indonesian Badan Penyelenggara Jaminan Kesehatan (BPJS), the national cost of treating cardiovascular disease have increased from 6,9 trillion rupiahs at 2015 to 7,4 trillion rupiahs at 2016.2
The major cause of cardiovascular diseases is atherosclerosis, a chronic pathological process marked by the accumulation of lipids in the endothelial wall, thickening the endothelial wall and reducing the lumen. Overtime atherosclerotic plaques become fibrotic and thin, increasing possibility of rupture and creating blood clots in important vessels such as coronary arteries.3 The biggest risk factor in the development of atherosclerosis is an imbalance of blood lipids, termed dyslipidemia. Atherogenic dyslipidemia, defined as a triad of increased low-density lipoprotein (LDL) particles, decreased high-density lipoprotein (HDL) and increased triglycerides, were found to be an important independent risk factor for cardiovascular diseases.4 Indeed, fibrate treatment aimed at markers of atherogenic dyslipidemia were shown to significantly reduce vascular event risk.5
Due to the prevalence of cardiovascular diseases, disease models should be designed for cardiovascular studies, particularly on dyslipidemia. Previous study by Harsa used rats fed with diets consisting of high fat content through oral gavage with promising results.6 However, previous studies revealed the methods used, particularly the oral gavage procedure, produced unwanted side effects such as allergies on the rats. Thus the present study replaced the oral gavage procedure by mixing the contents
of the high-fat diet with standard rat feed and feeding it by normal methods, that is, by natural feeding.
The success of the high-fat diet in inducing dyslipidemia can be identified in the lipid profile parameters. Of particular importance are the total cholesterol content, triglycerides, HDL cholesterol and LDL cholesterol. The present study researched the effects of the high-fat diet on those lipid profile parameters.
MATERIALS AND METHODS
Standard rat feed was mixed with 30% pig fat and 20% egg yolks to produce the high-fat diet. Thus, 1kg standard feed is mixed with 300g pig fat and 200g egg yolk to produce 1,5kg high-fat diet. Subsequent high-fat diets were produced using these composition.
Six three-months-old Wistar rats were divided into two groups of three rats each: the normal-fed group (NF) and the high-fat fed group (FF). Each rats were fed for 6 weeks according to the following regimen: 10g standard rat feed for NF group and 15g high-fat diet (±10g standard feed + 3g pig fat + 2g egg yolk) for the FF group.
After the treatment period, the rats were anesthesized with ketamine 44-100 mg/kgBW IM prior to blood extraction. Blood sample was arvested via the retroorbital vein, then labeled accordingly. The blood samples were then analyzed for their total cholesterol by CHOD-PAP method, triglycerides by GPO-PAP method, and HDL by homogeneous enzymatic colorimetric assay. Blood sample analysis were done at the Clinical Pathology Laboratory of Sanglah General Hospital, Denpasar. LDL were approximated using Friedewald’s equation: LDL=TC-HDL-(TG/5). Total cholesterol, triglycerides, HDL and LDL cholesterols were measured in mg/dL.
Data was collected and measured quantitatively. Statistical analysis was done via computer software. The data was analysed for the normality of distribution and central tendency. Statistical significance were calculated using t-test method. The results were found to be statistically significant if the p value is below 0.05. RESULTS
The mean of total cholesterol levels was 55.0 mg/dL in NF group and 92.67 mg/dL in FF group (p=0.027). The mean of triglyceride levels was 78.3 mg/dL in NF group and 171.67 mg/dL in FF group (p=0.014). The mean of HDL cholesterol levels was 32.00 mg/dL in NF group and 43.00 mg/dL in FF group (p=0.422). The mean of LDL cholesterol levels was 7.33 mg/dL in NF group and 15.33 mg/dL in FF group (p=0.437). The results were presented in Table 1 and Figure 1.
Table 1. Mean of lipid profile parameters after treatment. *p<0.05
|
Lipid Profile Parameter |
NF (n=3) |
FF (n=3) |
p |
|
Total cholesterol (mg/dL) |
55.00 |
92.67 |
0.027* |
|
Triglycerides (mg/dL) |
78.33 |
171.67 |
0.014* |
|
HDL cholesterol (mg/dL) |
32.00 |
43.00 |
0.422 |
|
LDL cholesterol (mg/dL) |
7.33 |
15.33 |
0.437 |
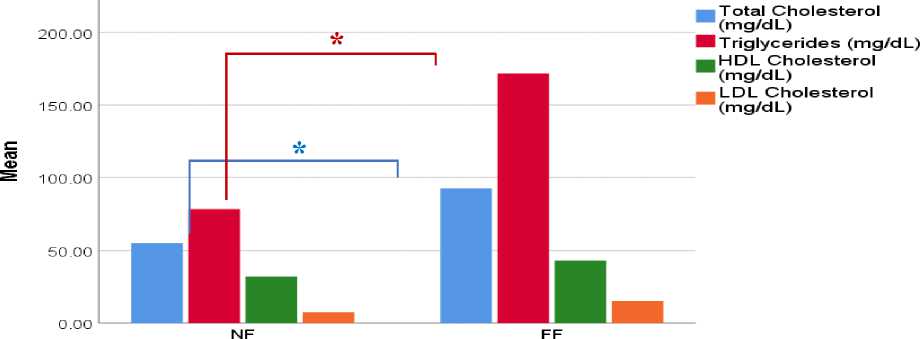
Treatment Sroup
Figure 1. The mean of total cholesterol, triglycerides, HDL and LDL cholesterol between treatment groups. *p<0.05
DISCUSSION
90% of dietary fats are triglycerides. After the triglycerides are broken down into its fatty acids in the intestinal lumen, the fatty acids are resynthesized into triglycerides within enterocytes. Along with cholesterol and lipoproteins, mostly apoB48, triglycerides is packaged into chylomicrons and sent to peripheral tissues via lymphatic vessels.7
Upon arriving to peripheral tissues, the triglycerides in chylomicrons are hydrolyzed by lipoprotein lipase (LPL) and broken back into their fatty acid constituents, and the fatty acids enter the peripheral cells. The rest are smaller, denser particles called chylomicron remnants. The remnants acquire cholesteryl esters from HDL and travels to the liver, where they are taken into the liver via lipoprotein-receptor related protein (LRP) to be recycled into their constituent apolipoproteins.8
Unlike triglycerides which are primarily obtained from dietary fats, cholesterol level is tightly regulated in the body. Cholesterol is an important molecule for the structural integrity of mammalian cell membranes and the precursor of steroid hormones, vitamin D, and bile acids. Cholesterols are acquired by a balance of dietary intake and de novo synthesis. Virtually all human cells have the capacity to synthesize cholesterol, however the major site of cholesterol synthesis happens in the liver. Cholesterols are synthesized from acetyl-coenzyme A through a series of pathways involving the rate-limiting enzyme HMG-CoA reductase.9
The regulation of cholesterol synthesis mainly happens via gene expression. Of particular importance is the liver X receptor (LXR). When oxysterols accumulate as a result of increasing intracellular cholesterol, LXR induces transcription of genes which reduces cholesterol to maintain homeostasis. These genes include ABCA1 and ABCG1 genes which facilitates cholesterol efflux via HDL, and sterol regulatory binding proteins (SREBPs) which reduces the expression of HMG-CoA reductase and ApoB(LDL) receptors.10 When dietary intake are high the rate of endogenous synthesis decreases, and vice versa.
In the present study, rats were fed with diet high in fat content to see its effects on plasma lipids concentration. After 6 weeks of feeding, total cholesterol increases significantly (70%). However, LDL and HDL cholesterol did not differ significantly. Instead, there was an almost two-fold increase (97%) in triglycerides. This is consistent with the fact that most triglycerides are obtained via diet. Cholesterol, on the other hand, has regulatory mechanism which ensures adequate cholesterol levels to maintain normal bodily functions while prevents excess cholesterol accumulation. It is possible that this regulatory mechanism functions extremely well in the subjects that feeding dietary fats through natural feeding alone was not adequate to impair the cholesterol regulatory mechanism. Activation of LXR by accumulation of intracellular cholesterol increases transcription of ABCA1, ABCG1 and SREBPs, which initiates the pathway to decrease endogenous cholesterol synthesis and maintaining LDL and HDL at similar levels even with high fat feeding.
However, previous studies which induced dyslipidemia on rats have been successful in significantly increasing LDL ad lowering HDL, albeit with different methods. The study by Harsa used the same composition of high-fat diet, namely pig fat and egg yolks, however the pig fat and egg yolk are made into a suspension with the use of carboxymethylcellulose (CMC) and fed by oral gavage. The study found significant increase of LDL and decrease of HDL after 4 weeks of feeding.6 Another study by Zhang et al. also found significant increase of LDL and decrease of HDL, although the study used pure cholesterol instead of high-fat diet from natural sources.11
CONCLUSION
The current regimen of high-fat diet feeding significantly increased total cholesterol levels, particularly in triglycerides. Other lipid profile parameters such as HDL and LDL were found to be statistically indifferent between treatment groups. This does not agree with the definition of atherogenic dyslipidemia. Thus, the present model is not
yet appropriate as a model for dyslipedimia, particularly for future studies on atherosclerosis.
Future studies should use a more vigorous approach to induce dyslipidemia, such as feeding a high-fat suspension via oral gavage, or using pure cholesterol. The levels of gene expression involved in cholesterol regulation should also be measured, to assess the integrity of the cholesterol regulatory system.
REFERENCES
-
1. WHO. Cardiovascular diseases (CVDs) [Internet].
2017 [cited 2018 Jan 23]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
-
2. Depkes RI. Riset Kesehatan Dasar 2013 [Internet].
2014. Available from:
http://www.depkes.go.id/resources/download/genera l/Hasil Riskesdas 2013.pdf
-
3. Moore KJ, Tabas I. Macrophages in the Pathogenesis of Atherosclerosis. Cell. Elsevier; 2011;145(3):341– 55.
-
4. Musunuru K. Atherogenic dyslipidemia:
Cardiovascular risk and dietary intervention. Lipids. 2010;45(10):907–14.
-
5. Lee M, Saver JL, Towfighi A, Chow J, Ovbiagele B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: A metaanalysis. Atherosclerosis. Elsevier Ireland Ltd;
2011;217(2):492–8.
-
6. Harsa IMS. Efek Pemberian Diet Tinggi Lemak terhadap Profil Lemak Darah Tikus Putih (Rattus norvegicus). J Ilm Kedokt. 2014;3(1):21–8.
-
7. Dominiczak MH, Priest M, Kulkarni U V, Broom JI. Digestion and Absorption of Nutrients: The
Gastrointestinal Tract. In: Medical Biochemisitry. 4th ed. Saunders Elsevier; 2014. p. 121–2.
-
8. Dominiczak MH. Lipoprotein Metabolism and Atherogenesis. In: Medical Biochemisitry. 4th ed. Saunders Elsevier; 2014. p. 219.
-
9. Dominiczak MH, Beastall G, Wallace AM. Biosynthesis of Cholesterol and Steroids. In: Medical Biochemisitry. 4th ed. Saunders Elsevier; 2014.
-
10. Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol.
2010;204(3):233–40.
-
11. Zhang L, Fang G, Zheng L, Chen Z, Liu X. Hypocholesterolemic effect of capsaicinoids in rats fed diets with or without cholesterol. J Agric Food Chem. 2013;61(18):4287–93.
http://ojs.unud.ac.id/index.php/eum
doi:10.24843.MU.2022.V11.i10.P04
24
Discussion and feedback