THE EFFECT OF PANDAN WANGI ROOTS ETHANOL EXTRACT (Pandanus amaryllifolius Roxb.) ON THE DEGREE OF PLASMODIUM BERGHEI PARASITEMIA IN VIVO
on

ISSN: 2597-8012 JURNAL MEDIKA UDAYANA, VOL. 13 NO.02, FEBRUARI, 2024


Received: 2023-10-05 Revision: 2023-12-28 Accepted: 22-01-2024
THE EFFECT OF PANDAN WANGI ROOTS ETHANOL EXTRACT (Pandanus amaryllifolius Roxb.) ON THE DEGREE OF PLASMODIUM BERGHEI PARASITEMIA IN VIVO
Ni Made Kumbha Mella Saraswati Devi1, Dewa Ayu Agus Sri Laksemi2, Ni Luh Putu
Eka Diarthini2, I Made Sudarmaja2
1 Bachelor of Medicine and Medical Proffesion, Faculty of Medicine, Udayana University
Correspondence e-mail: srilaksemi@unud.ac.id
ABSTRACT
Malaria is an disease caused by Plasmodium genus and transmitted by female Anopheles mosquitoes via bites. The occurrence of antimalarial drug resistance in various places, such as Chloroquine and Artemisinin Combination Therapy, be a challenge for researchers to find new herbal and effective alternative antimalarial drugs. Pandan Wangi roots (Pandanus amaryllifolius Roxb.) contain compounds with antimalarial properties. This study aims to determine the effect of Pandan Wangi roots ethanol extract at doses of 1, 100, and 1,000 mg/kgBW (Body Weight on the degree of Plasmodium berghei parasitemia in vivo. This study employs an experimental in vivo method using the Post-Test Only Controlled Group Design. Sample of 24 mice were divided into one negative control group and three treatment groups. The data analysis technique employed is One Way ANOVA and Post Hoc Test. In this study, the mean degree of parasitemia in negative control group was 32.60%, treatment group with dose of 1 mg/kgBW was 22.27%, treatment group with dose of 100 mg/kgBW was 18.49%, and treatment group with dose of 1,000 mg/kgBW was 14.88%. The One Way ANOVA analysis resulted in p-value of 0.001, which is < 0.05, indicating a significant difference in the average degree of parasitemia among the three treatment groups. Thus, the effect of Pandan Wangi roots ethanol extract has antimalarial activity. Administration dose of 1,000 mg/kgBW proved to be the most effective in suppressing the growth of Plasmodium berghei parasitemia.
Keywords : Pandanus amaryllifolius Roxb., Antimalarial, Plasmodium berghei, Degree of Parasitemia
INTRODUCTION
Malaria is an disease caused by Plasmodium genus and transmitted by female Anopheles mosquitoes via bites.1 It has become one of the most severe infectious diseases, threatening nearly half of the world's population, particularly in regions like Africa.2 As a result, malaria poses a life-threatening risk and a significant global health threat. Annually, approximately two billion people are at risk of contracting malaria.3 Looking at malaria elimination in Indonesia, none of the provinces in the eastern part of the country has achieved elimination. Papua has the highest Malaria Morbidity Rate (Annual Parasite Incidence/API) at 52.99 per 1,000 population.4 Symptoms of this disease include fluctuating fever, chills, diaphoresis, myalgia, nausea, vomiting, headache, muscle pain, and stiffness.5
Resistance to antimalarial drugs, such as chloroquine, has been observed in most parts of the world, including Africa.6 Resistance to artemisinin in Plasmodium sp infections7 presents a challenges for researchers to discover new and effective selective antimalarial drugs.8
Given the reality of resistance to chloroquine and artemisinin, efforts are needed to develop new herbal alternative antimalarial drugs. One such attempt involves
using the pandan wangi plant (Pandanus amaryllifolius Roxb.), which is readily available in tropical and subtropical regions like Indonesia. This plant is known for its pleasant fragrance, containing chemical compounds such as alkaloids, saponins, steroids, flavonoids, terpenoids, tannins, polyphenols, and coloring agents.9
MATERIALS AND METHODS
This study employs an experimental in vivo method using the Post-Test Only Controlled Group Design conducted from March to August 2023. The sample used in this study consisted of 24 male Balb/c strain mice that had been infected with Plasmodium berghei. The selection of these mice was based on inclusion requirements, specifically healthy male Balb/c strain mice weighing 25-30 grams and aged approximately 2-3 months. Exclusion criteria included mice that died or went missing. After meeting the criteria, the mice were divided into one negative control group and three treatment groups. The negative control group received distilled water and ethanol. In contrast, the three treatment groups were given ethanol extract suspension of pandan wangi roots at doses of 1 mg/kgBW, 100 mg/kgBW, and 1,000 mg/kgBW, respectively. The antimalarial activity in
this study was measured by calculating the percentage of parasitemia in each mice samples infected with Plasmodium berghei using The Peters' 4-day suppressive test method. The research involved the utilization of Plasmodium berghei, which was acquired from the Department of Parasitology, Faculty of Medicine at Universitas Udayana. This study has obtained ethical clearance with the number 786/UN14.2.2.VII.14/LT/2023 issued by the Research Ethics Commission (KEP) of the Faculty of Medicine, Universitas Udayana.
Preparation of Ethanol Extract from Pandan Wangi Roots (Pandanus amaryllifolius Roxb.)
Fresh pandan wangi roots are cleaned thoroughly using running water. Subsequently, they are blended until they turn into powder. A total of 1 kg of pandan wangi root powder is then filtered or sieved again to obtain a finer powder, which is then dried at room temperature for 8 days (with an indicator of water content <10%).9 The dried powder sample is then macerated with 80% ethanol for 1 day. It is shaken for 3 hours using a shaker at a speed of 120 rpm. Afterward, it is filtered to get the filtrate and concentrated using a rotary evaporator to obtain a concentrated extract.10 The extract is administered to the treatment groups according to the predetermined daily doses for 4 consecutive days (H0-H3) using a probe.
Preparation and Grouping of Test Animals
This study utilized a sample of 24 male Balb/c strain mice that had been infected with Plasmodium berghei, each weighing between 25-30 grams and aged approximately 2-3 months. All mice must meet the inclusion criteria. The total number of cages required corresponds to the number of mice groups, which is four cages, with each cage housing six mice. Each mice is labeled differently for easy identification during the research. The mice are divided into one group as the negative control (K) and three as treatment groups (P1, P2, P3) with their respective predetermined doses.
Plasmodium berghei is first propagated within the bodies of 5 donor mice and left undisturbed for 2-3 days. If the parasitemia level in the donor mice reaches >10%, they can be inoculated or transferred to the test mice. The inoculation process is carried out intraperitoneally with a volume of 0.2 cc.11 The negative control group (K) was given distilled water and ethanol. In contrast, the treatment groups (P1, P2, and P3) were administered ethanol extract of pandan wangi roots at doses of 1 mg/kgBW, 100 mg/kgBW, and 1,000 mg/kgBW, respectively. The extract is administered orally to the mice for 4 days (H0-H3) using a probe.
Preparation of Thin Blood Smears and Observation of Parasitemia Degree
The observation of parasitemia degree was conducted by preparing thin blood smears. A drop of blood is collected from the mice's tail, and the smear is fixed with methanol and then air-dried. After air-drying at room temperature, the smear is stained with 10% Giemsa for 30 minutes, followed by washing with running water and air-drying again at room temperature. The examination of thin blood smears is conducted utilizing a light microscope at a magnification of 1,000 times. The calculation of the percentage of parasitemia is done by tallying the count of Plasmodium berghei-infected erythrocytes per 1,000 erythrocytes.12 Subsequently, the percentage of parasitemia and the inhibition percentage of the test substance against parasite growth are calculated using the following formula:13
Number of Infected Erythrocytes × 100% Parasitemia Percentaqe (%) =--------———:-----------------
Total Erythrocyte Count
Inhibition Percentage (%) = 100% — — X 100%
-
- Xp (Parasitemia of treatment group) =
-
% Parasitemia of treatment group (dn – d0)
-
- Xk (Parasitemia of control group) =
% Control parasitemia (dn – d0)
-
- [(Xp/Xk) x 100% = Percentage Parasite Growth
-
- (dn – d0) = Percentage parasitemia on day-n minus
percentage parasitemia on day-zero
Data Analysis
The data in this study were analyzed using the Software Statistical Package for the Social Science (SPSS) version 26. The data analysis tools in this research are as follows:
-
1. To determine the antimalarial activity of ethanol extract of pandan wangi roots (Pandanus
amaryllifolius Roxb.), a descriptive analysis was conducted.
-
2. To assess the differences in parasitemia levels for doses of 1 mg/kgBW, 100 mg/kgBW, and 1,000
mg/kgBW of ethanol extract of pandan wangi roots (Pandanus amaryllifolius Roxb.) compared to the negative control group, a two-sample mean difference test was employed. This was carried out using Post Hoc Test through Tukey analysis at a significance level (α) of 5%.
-
3. To examine the differences in parasitemia levels among doses of 1 mg/kgBW, 100 mg/kgBW, and 1,000 mg/kgBW of ethanol extract of pandan wangi roots (Pandanus amaryllifolius Roxb.), a One-Way Analysis of Variance (One Way ANOVA) was utilized at a significance level (α) of 5%. The One Way ANOVA test requires two assumptions, namely the normality of distribution and the homogeneity of variances. Therefore, before conducting the One Way
ANOVA test, the Shapiro-Wilk test was utilized to assess normality, while Levene's test was employed for checking homogeneity of variance.
RESULT
Antimalarial Activity Test
Observation of antimalarial activity was conducted by measuring the degree of parasitemia in each group, including K, P1, P2, and P3. The results of parasitemia degree measurements are presented in Table 1.
From Table 1, it can be observed that the highest average parasitemia occurred in the negative control group (K), reaching 32.60% with a parasite inhibition percentage (suppression) of 0.00%. In contrast, the lowest average parasitemia occurred in treatment group 3 (P3), amounting to 14.88% with a parasite inhibition percentage (suppression) of 54.36%.
Table 1. Results of Antimalarial Activity in Each Group
Antimalarial Activity
Group % Parasitemia %
± S.D. suppression
|
Control (-) |
32,60 ± 9,06 |
0,00 |
|
Treatment 1 |
22,27 ± 3,56 |
31,69 |
|
Treatment 2 |
18,49 ± 1,85 |
43,28 |
|
Treatment 3 |
14,88 ± 1,66 |
54,36 |
Examining mice blood smears involves observing red blood cells infected by Plasmodium berghei parasites through a microscope at a magnification of 1,000x. This is done to calculate the degree of parasitemia in each mice.
The results of blood smears in the negative control group (K), treatment 1 (P1), treatment 2 (P2), and treatment 3 (P3) are presented in Figures 1A, 1B, 1C, and 1D. Figures with arrow indicators point to mice’s red blood cells infected with Plasmodium berghei.
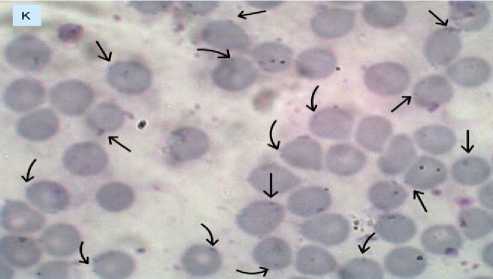
Figure 1A. Erythrocytes infected with Plasmodium berghei in the negative control group (K).
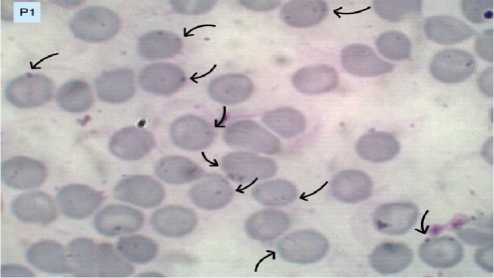
Figure 1B. Erythrocytes infected with Plasmodium berghei in treatment group 1 (P1).
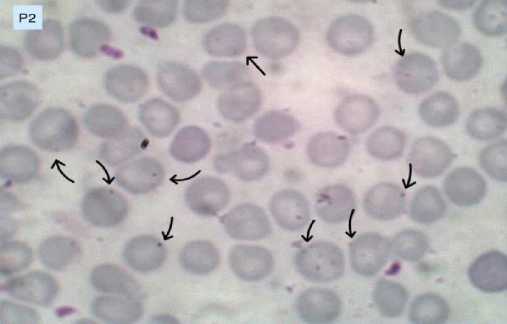
Figure 1C. Erythrocytes infected with Plasmodium berghei in treatment group 2 (P2).
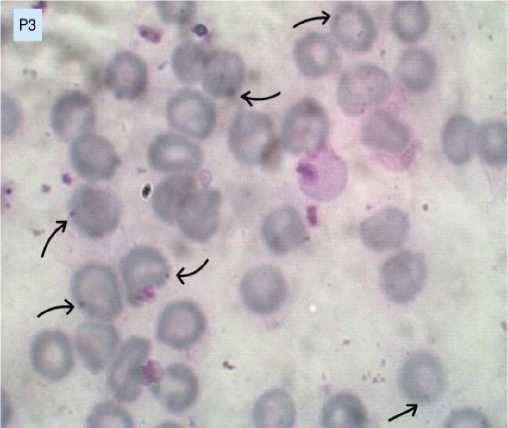
Figure 1D. Erythrocytes infected with Plasmodium berghei in treatment group 3 (P3).
Normality Test
The normality test used in this study is the Shapiro-Wilk test, with the rationale that the sample size in this research is only 24 (less than 50).14 Based on the results of the Shapiro-Wilk test, as shown in Table 2, a p-value > 0.05 was obtained. Therefore, it can be concluded that the degree of parasitemia in all four groups follows a normal distribution.
|
Table 2. Results of Normality Test using Shapiro-Wilk Test | ||||
|
Group |
Sample (n) |
Statistics |
df |
p-value |
|
Control (-) |
6 |
0,875 |
6 |
0,249 |
|
Treatment 1 |
6 |
0,928 |
6 |
0,563 |
|
Treatment 2 |
6 |
0,951 |
6 |
0,751 |
|
Treatment 3 |
6 |
0,823 |
6 |
0,094 |
Homogeneity Test
The test used to determine homogeneity in this study is Levene's test of homogeneity of variance, and the test results with a p-value > 0.05 can be observed in Table 3. It can be concluded that the variance of parasitemia degree in Treatment 1, Treatment 2, and Treatment 3 is homogeneous.
Table 3. Homogeneity Test
|
Variable |
Levene (Sig.) |
|
Parasitemia Degree |
0,111 |
Mean Difference Test between Negative Control and Each Treatment 1, Treatment 2, and Treatment 3
Based on the Post Hoc Test with Tukey analysis in Table 4, a p-value < 0.05 was obtained. Therefore, it can be concluded that there is a significant difference in the mean value of parasitemia degree between the negative control group and treatments 1, 2, and 3.
Table 4. Results of Tukey Analysis between Negative Control and Treatment 1, Treatment 2, and Treatment 3
|
Groups |
Mean Difference |
p-value |
|
K vs P1 |
10,33 |
0,010 |
|
K vs P2 |
14,11 |
0,001 |
|
K vs P3 |
17,71 |
0,000 |
One Way Analysis of Variance (one way ANOVA)
In one-way ANOVA analysis, there are two assumptions that must be fulfilled, namely that the data is normally distributed and has equal variances. In this study, both assumptions have been met, allowing the one-way ANOVA analysis to proceed. Based on the results of the one-way ANOVA test, a p-value < 0.05 was obtained. Therefore, it can be concluded that there is a significant difference in the mean value of parasitemia degree among
treatment 1, 2, and 3. The results of the One-Way ANOVA test can be observed in Table 5.
|
Table 5. One-Way ANOVA Test | ||||
|
Sum of Squares |
df |
Mean Square |
Sig. | |
|
Between |
163,791 |
2 |
81,895 |
0,001 |
|
Groups Within |
94,181 |
15 |
6,279 | |
|
Groups Total |
257,972 |
17 | ||
Post Hoc Test
Based on Table 6, the p-value (P1 vs P2) is < 0.05, indicating a significant difference in the mean parasitemia degree between treatment 1 and treatment 2. Similarly, the p-value (P1 vs P3) is < 0.05, signifying a significant difference in the mean parasitemia degree between treatment 1 and treatment 3. On the other hand, the p-value (P2 vs P3) is > 0.05, suggesting that the mean parasitemia degree between treatment 2 and treatment 3 is same, or there is no significant difference.
Table 6. Results of Tukey Analysis on the Mean Parasitemia Degree among Treatment 1, Treatment 2, and Treatment 3
|
Groups |
Mean Difference |
p-value |
|
P1 vs P2 |
3,78 |
0,049 |
|
P1 vs P3 |
7,38 |
0,000 |
|
P2 vs P3 |
3,61 |
0,061 |
DISCUSSION
The results of this study indicate that the administration of ethanol extract from pandan wangi roots (Pandanus amaryllifolius Roxb.) has been proven to have an inhibitory effect on the growth of Plasmodium berghei in male Balb/c mice. The administration of ethanol extract from pandan wangi roots in treatment 3 (P3) with a dose of 1,000 mg/kgBW has been proven to be the most effective in suppressing the parasitemia growth of Plasmodium berghei.
The ethanol extract of pandan wangi roots (Pandanus amaryllifolius Roxb.) administered to male Balb/c mice, which were already infected with Plasmodium berghei parasites, contains alkaloid compounds with lactone/alkaloid lactone groups9 compounds have been proven to have antimalarial activity when tested against Plasmodium falciparum in vitro.15 Theoretically, the lactone alkaloid have potential as antimalarials and their mechanism of action is similar to the aminoquinoline antimalarial drugs.9 Lactone alkaloids are among the alkaloids present in pandan wangi extract. These alkaloids act as antimalarials by inhibiting the detoxification of parasite haem in the food
vacuole, as well as acting as blood schizonticides and gametocides.16 Alkaloids are also known as compounds that can influence the immune system, acting as immunostimulants.9 In addition to alkaloids, pandan wangi root extract is also proven to contain active ingredients such as flavonoids, saponins, steroids, and terpenoids, which not only have the potential as antimalarial secondary metabolites but may also act as immunomodulators.15
Another study conducted by Irmayanti and colleagues, in which they investigated the effectiveness of pandan wangi root extract as an antimalarial on the eosinophil counts with concentrations of 6.5%, 13%, and 26%, proved to reduce parasitemia levels more effectively than the negative control group, with average parasitemia degrees of 10.6%, 9.4%, and 6.8%, respectively.16 Another study examining the effectiveness of pandan wangi root extract as an antimalarial on the neutrophil count was conducted by Sari and colleagues. They concluded that a concentration of 26% pandan wangi root extract demonstrated the best antimalarial activity, characterized by a decrease in parasitemia levels and an increase in neutrophils.15 Furthermore, supporting this research is a study by Mustofa and colleagues that investigated the effectiveness of methanol extract from pandan wangi roots on the lymphocyte count. The study concluded that the optimal extract concentration was 26%, exhibiting antimalarial activity with an 8.6% reduction in parasitemia levels.9
Various herbal plants have been studied to prove the effectiveness of alkaloid compounds as antimalarials, such as papaya leaf extract (Carica papaya), guava leaf extract (Psidium guajava),17 merkubung leaf extract (Macaranga gigantea),18 gempol leaf extract (Nauclea orientalis L.),19 and noni fruit extract (Morinda citrifolia L.).20 Based on the previous studies outlined above, it is evident that many herbal plants have the potential as antimalarials.
CONCLUSIONS AND SUGGESTIONS
Ethanol extract of pandan wangi roots (Pandanus amaryllifolius Roxb.) exhibits antimalarial activity. The highest average parasitemia degree occurred in the negative control group (K), reaching 32.60%, with a parasite inhibition percentage (suppression) of 0.00%. On the other hand, the lowest average parasitemia degree occurred in the mice of treatment group 3 (P3), at 14.88%, with a parasite inhibition percentage (suppression) of 54.36%. The administration of ethanol extract from pandan wangi roots (Pandanus amaryllifolius Roxb.) at a dose of 1,000 mg/kgBW proved to be the most effective in suppressing the growth of Plasmodium berghei parasitemia.
Further research on the effectiveness of doses of ethanol extract from pandan wangi roots (Pandanus amaryllifolius Roxb.) is needed. This is because, considering that the most effective dose in suppressing the growth of Plasmodium berghei parasitemia (administration of a dose
of 1,000 mg/kgBW) in this study only achieved a suppression (parasite inhibition percentage) of 54.36%, it is hoped that a more effective dose with more significant suppression percentage than 54.36% can be identified through further research.
REFERENCES
-
1. Talapko J, Škrlec I, Alebić T, Jukić M, Včev A. Malaria: The Past and the Present. Microorganisms. 2019 Jun;7(6).
-
2. Phillips MA, Burrows JN, Manyando C, van Huijsduijnen RH, Van Voorhis WC, Wells TNC. Malaria. Nat Rev Dis Prim. 2017 Aug;3:17050.
-
3. Buck Emily, Finnigan. Nancy A. Malaria [Internet]. Treasure Island (FL): StatPearls [Internet].; 2023. Available from:
https://www.ncbi.nlm.nih.gov/books/NBK551711/
-
4. Kemenkes RI. Profil Kesehatan Indonesia 2018 [Internet]. Health Statistics. Kemenkes; 2019. 207 p. Available from:
https://www.kemkes.go.id/downloads/resources/downl oad/pusdatin/profil-kesehatan-indonesia/profil-kesehatan-indonesia-2018.pdf
-
5. Juniantara IKA, Laksemi DAAS, Damayanti PAA, Diarthini NLPE. Uji Aktivitas Buah Mahkota Dewa (Phaleria Macrocarpa) Sebagai Antimalaria Pada
Mencit Yang Diinfeksi Plasmodium Berghei. J Med Udayana [Internet]. 2022;11(12):85–90. Available
from: http://ojs.unud.ac.id/index.php/eum85
-
6. Daily JP, Minuti A, Khan N. Diagnosis, Treatment, and Prevention of Malaria in the US: A Review. JAMA. 2022 Aug;328(5):460–71.
-
7. WHO. Report on antimalarial drug efficacy, resistance and response 10 years of surveillance (2010-2019). 2020.
-
8. Marliana E, Saleh C, Hendra M. Antioxidant and Antimalarial Activities of Phenolic Compounds from Leaves of Macaranga beccariana Merr. J Kim MULAWARMAN. 2018 May 30;15:106.
-
9. Mustofa D, Kahtan MI, Natalia D, Zakiah M, Widiyantoro A. Efektivitas ekstrak metanol akar
pandan wangi (Pandanus amaryllifolius Roxb.)
Sebagai antimalaria terhadap jumlah limfosit dalam darah mencit (Mus musculus) yang diinfeksi Plasmodium berghei. Intisari Sains Medis. 2019;10(2):489–96.
-
10. Ngibad K. Efektivitas Kombinasi Ekstrak Etanol Daun Bunga Matahari dan Tanaman Anting-Anting sebagai Antimalaria Secara In Vivo. J Farm Galen (Galenika J Pharmacy). 2019;5(1):12–9.
-
11. Dila G, Kahtan MI, Widiyantoro A. Efektivitas Ekstrak Metanol Akar Pandan Wangi (pandanus amaryllifolius roxb.) Sebagai Antimalaria Terhadap Jumlah Parasitemia dan Monosit Dalam Darah Mencit
(mus musculus) yang Diinfeksi Plasmodium berghei. Biomedika. 2020;12(1).
-
12. Syaifudin M, Irma I, Ramadhani D, Teknologi P, Radiasi M, Nuklir BT, et al. Optimalisasi Pewarnaan Giemsa Pada Apusan Darah Tipis Terinfeksi
Plasmodium berghei Untuk Mendukung
Pengembangan Vaksin Malaria Iradiasi hasil.
Keberhasilan tersebut ditandai membantu menurun
dari waktu ke waktu , jika pada World Health Organization (WHO). J Biotek Medisiana Indones. 2018;Vol.7:77–84.
-
13. Taek M. Aktivitas Antimalaria Ekstrak Strychnos Ligustrina Sebagai Obat Tradisional Antimalaria di Timor Dalam Uji In-Vivo pada Mencit yang Terinfeksi Plasmodium berghei. 2018.
-
14. Quraisy A. Normalitas Data Menggunakan Uji Kolmogorov-Smirnov dan Saphiro-Wilk: Studi kasus penghasilan orang tua mahasiswa Prodi Pendidikan Matematika Unismuh Makassar. J-HEST J Heal Educ Econ Sci Technol. 2022 Jul 31;3:7–11.
-
15. Sari PR, Kahtan MI, Widiyantoro A. Efektivitas Ekstrak Akar Pandan Wangi (Pandanus amaryllifolius Roxb.) sebagai Antimalaria terhadap Jumlah Neutrofil dalam Darah Mencit (Mus musculu) yang diinfeksi Plasmodium berghei Program Studi Kedokteran , FK UNTAN Program Studi Kimia , FMIPA UNT. J Cerebellum. 2019;5(3B):1433–41.
-
16. Irmayanti S, Widiyantoro A, Kahtan MI. Efektivitas Ekstrak Akar Pandan Wangi (Pandanus amaryllifolius Roxb.) sebagai Antimalaria terhadap Jumlah Eosinofil pada Mencit (Mus musculus) yang diinfeksi Plasmodium berghei. J Cerebellum [Internet]. 2017;3(1):709–17. Available from:
https://jurnal.untan.ac.id/index.php/jfk/article/view/235 79/18505
-
17. Arifuddin M, Bone M, Rusli R, Kuncoro H, Ahmad I, Rijai L. Aktivitas Antimalaria Penghambatan Polimerisasi Heme Ekstrak Etanol Daun Jambu Biji (Psidium guajava) dan Daun Pepaya (Carica papaya). J Ilm Ibnu Sina Ilmu Farm dan Kesehat. 2019 Jun 2;4:235–43.
-
18. Muhaimin M, Yusnaidar Y, Syahri W, Latief M, Chaerunisaa A. Microencapsulation of Macaranga gigantea Leaf Extracts: Production and
Characterization. Pharmacogn J. 2020 Jun 18;12:716– 24.
-
19. Budiarti M, Maruzy A, Rk N, Brotojoyo E. Aktivitas Antimalaria Daun Gempol (Nauclea orientalis (L.) L) terhadap Plasmodium falciparum Antimalarial Activity from Gempol (Nauclea orientalis (L.) L) Leaves Againts Plasmodium falciparum. Media Penelit dan Pengemb Kesehat. 2020 Sep 30;30:135–46.
-
20. Sogandi S, Rabima R. Identifikasi Senyawa Aktif Ekstrak Buah Mengkudu (Morinda citrifolia L.) dan Potensinya sebagai Antioksidan. J Kim Sains dan Apl. 2019 Sep 30;22:206.

http://ojs.unud.ac.id/index.php/eum
doi:10.24843.MU.2024.V13.i02.P13
P a g e 73
Discussion and feedback