Prevalence and Hemodynamic Outcome of Dengue Shock Syndrome in Children Attending the Department of Pediatrics, Dr. Soetomo General Hospital
on
ARTIKEL PENELITIAN

ESSENTIAL:Essence of Scientific Medical Journal (2020), Volume 18, Number 1:12-16
P-ISSN.1979-0147, E-ISSN. 2655-6472
PENELITIAN
PREVALENCE AND HEMODYNAMIC OUTCOME OF DENGUE SHOCK SYNDROME IN CHILDREN ATTENDING THE DEPARTMENT OF PEDIATRICS, DR. SOETOMO GENERAL HOSPITAL
Fenska Seipalla,1 Ira Dharmawati,1 Sundari Indah Wiyasihati,1
ABSTRACT
The prevalence of dengue infection has increased markedly worldwide. Dengue shock syndrome (DSS) is a severe manifestation of dengue virus infection. Higher mortality of DSS was found in children. This study’s aim was to portray prevalence and hemodynamic outcome in children attending the department of pediatric in Dr. Soetomo General Hospital. A Descriptive Retrospective study of children aged <15 years old with DSS was performed and evaluated from 2013-2016. The samples were divided into 5 groups, aged <1 years old, 1-2 years old, 3-5 years old 6-11 years old and 12-15 years old. Data were taken secondarily and calculated with Microsoft Excel 2010. The most common findings were prevalence of DSS in 6-11 years old group and mortality rate in <1 years old group. This can be seen as in the hemodynamic outcome; the average HR was higher in aged group 1-2 years old while RR was higher in group under 1-year-old. Meanwhile, the average blood pressure, PP, and MAP were lower in children under 2 years old. The hemodynamic outcome varies on each group based on age. Incidence of DSS remained high in older children but mortality rates were high in younger children.
Keywords: Dengue Shock Syndrome, Pediatrics, Hemodynamic, Prevalence, Indonesia
1 Department of Physiology, Faculty of Medicine, Universitas Airlangga
INTRODUCTION
In this era of globalization, as many as 2.5 billion people or around 40% of the world's population live in areas of risk of dengue fever infection.[1] Dengue Hemorrhagic Fever (DHF) is an endemic outbreak that occurs in approximately 100 countries worldwide.[2] Class III and IV of DHF classification is included in Dengue Shock Syndrome (DSS). World Health Organization (WHO) estimates that about 50 - 100 million people were reported to be infected with the dengue virus, including about 500,000 cases of DHF / DSS per year with a 2.5% Case Fatality Rate (CFR) caused by DHF /DSS.[1]. Morbidity and mortality rates of dengue cases are more common in children. DHF mostly are found in tropical and subtropical regions. Indonesia has the highest incidence of dengue fever infection in Southeast Asia. In 2014, until mid-December recorded dengue fever cases in 34 provinces in Indonesia are as many as 71,668 people, and 641 of them died.[3] In Dr. Cipto Mangunkusumo National Central General Hospital Jakarta, there were 37.3% of shock cases due to DSS.[4]
Dengue virus infection can be dangerous and cause mortality if it’s not treated properly. This is due to the occurrence of hypovolemic shock in patients with DSS.[5] The theory of shock is caused by an immune complex that causes platelet aggregation and complement activation.[6] Platelet aggregation leads to Disseminated Intravascular Coagulation (DIC) which causes coagulation time to decrease and causes bleeding. In addition, platelet aggregation will be destroyed by the RES system that will result in the occurrence of thrombocytopenia that leads to bleeding. Whereas, complement activation will produce cytokines that can increase capillary permeability causing plasma infiltration.[7] In the case of DSS, plasma bleeding and inflammation that occurs will affect the body's hemodynamic system. Therefore, it is necessary to monitor early hemodynamic disorders that happened in patient with DSS. In this study, the hemodynamic parameters used were Heart Rate (HR), Blood Pressure (BP), Pulse Pressure (PP), Mean Arterial Pressure (MAP), and Respiratory Rate (RR).
METHODS
This study used a retrospective descriptive method to study the prevalence and hemodynamic outcome in DSS patient. The sample in this study were DSS patients aged ≤15 years that admitted to the Department of Pediatrics of Dr. Soetomo General Hospital from 1 January 2013 until 31 December 2016. The data were collected from the medical record center of Dr. Soetomo General Hospital and were analyzed using SPSS Statistics 24. The inclusion criteria include children aged undr 15 years old, diagnosed with DSS in department of Pediatrics, Dr. Soetomo General Hospital. The exclusion criteria include uncompleted medical record.
RESULTS
Despite the higher prevalence of dengue infection in Indonesia, in this study shows that the total number of patients that has been diagnosed with DSS is 44 cases from 2013 until 2016 at Dr. Soetomo General Hospital. The cases of DSS showed a decreasing trend. The majority of cases occurred in 2013 following by the mortality. The lowest prevalence occurred in 2014 with total number 6 cases while no mortality was reported in 2016 (Figure 1.).
The distribution of age shows that older patients (>3 years-old) has higher incidences compare to the younger patients (<3 years-old). However, higher mortality was found in children under 1-year-old (67%) (Table 1.).
Out of the 44 cases, only 35 cases that included in the following because of incomplete data of some laboratories’ information in the medical record. The clinical and laboratory characteristics at admission are shown in Table 2. Patients with DSS were mostly women with short duration of hospitalization. In addition, pleura effusions and low platelet are more likely to be found in patients who presented shock. Regarding the hemodynamic outcome (Table 3.), data were presented as the mean ± SD (Min-Max). This study found only 1 case of DSS in aged under 1-year-old. The hemodynamic parameter of this study was commonly used to assess patient condition by clinicians in emergency situation. The worst hemodynamic outcome was shown in younger
group. The average HR was higher in aged group 1
2 years old while RR was higher in group under 1-
year-old. The average of blood pressure, PP, and MAP were lower in children under 2 years old.
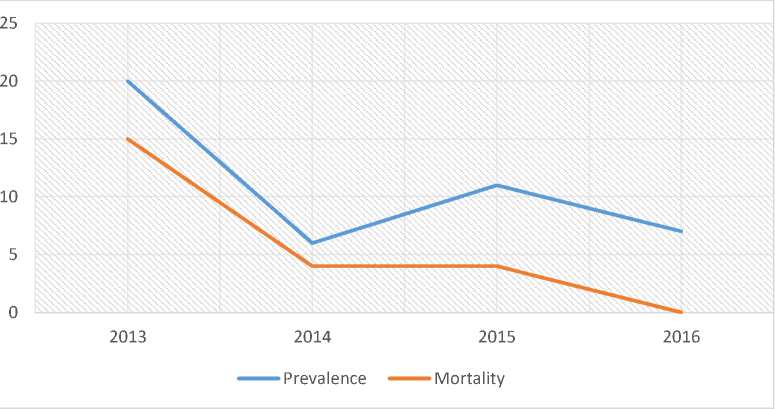
Figure 1. Prevalence and mortality of Dengue Shock Syndrome in Department of Pediatrics, Dr. Soetomo General Hospital from the year 2013 to 2016
Table 1. Prevalence and mortality of Dengue Shock Syndrome based on aged group
|
Age (y) |
Prevalence (n) |
Mortality (n, %) |
|
<1 |
3 |
2 (67) |
|
1-2 |
2 |
1 (50) |
|
3-5 |
17 |
9 (53) |
|
6-11 |
18 |
10 (56) |
|
12-15 |
4 |
1 (25) |
Table 2. General Characteristics, clinical parameters, and laboratory parameters among patients with Dengue Shock Syndrome
|
Characteristics |
N |
N/Median |
Interquartile Range (IQR)/frequency (%) |
|
Age |
35 |
5.5 |
(4-8.25) |
|
Male |
35 |
16 |
(45.7) |
|
Duration of hospitalization (days) |
35 |
3 |
(1-5) |
|
Clinical Parameters | |||
|
Fever Duration (days) |
35 |
5 |
(4-5) |
|
Pleura Effusion |
35 |
18 |
(51.4) |
|
GI Bleeding |
35 |
11 |
(31.4) |
|
CRT >2 |
35 |
17 |
(48.6) |
|
laboratory Parameter | |||
|
Hemoglobin, g/dL |
35 |
14.4 |
(12.2-15.4) |
|
Hematocrit, % |
35 |
42.7 |
(34.9-45.6) |
|
WBC, ×103∕μL |
35 |
6.72 |
(5-12.8) |
|
Platelet count, ×103∕μL |
35 |
34 |
(19-65) |
|
AST, IU/L |
34 |
445 |
(191.8-972.8) |
|
ALT, IU/L |
34 |
124 |
(70.5-356.8) |
Table 3. Hemodynamic outcome among patients with Dengue Shock Syndrome
|
Age (y) |
HR (beats/min) |
RR (breaths/min) |
SP (mmHg) |
DP (mmHg) |
PP (mmHg) |
MAP (mmHg) |
|
<1 |
120 |
50 |
80 |
60 |
20 |
67 |
|
1-2 |
140±28 (120-160) |
44±19 (30-57) |
85±7 (80-90) |
55±21 (40-70) |
30±28 (10-50) |
65±12 (57-73) |
|
3-5 |
131±25 (96-178) |
38±12 (24-60) |
89±12 (70-120) |
62±10 (50-80) |
28±8 (20-40) |
71±10 (57-93) |
|
6-11 |
122±17 (80-151) |
35±14 (20-60) |
95±13 (80-120) |
66±10 (50-80) |
29±7 (20-40) |
75±10 (60-93) |
|
12-15 |
104±30 (72-132) |
30±2 (28-32) |
103±6 (100-110) |
73±6 (70-80) |
30±10 (20-40) |
83±3 (80-87) |
DISCUSSION
Data show difference with the Incidence Rate (IR) of DHF cases in Indonesia where 2015 has the highest IR followed by 2013 and 2014. Meanwhile, the lowest CFR occurred in 2013.[8] This can be different because the report from Indonesia’s Ministry of Health included all age groups and does not differentiate data collected from children and adult. The report also does not differentiate between patients who presented shock and non-shock in dengue cases.
The distribution of age shows that that older children have a higher prevalence of DSS. Another study also shows patients > 6 years having a greater risk of DSS than age ≤6 years.[9] In Haiti, as many as 85% of children aged 6-13 years have DENV antibodies.[10] In Thailand, 55 children aged ≤15 years were diagnosed with DSS out of 165 children diagnosed with dengue.[11] Contradicted with the prevalence, the higher mortality was more likely to be found in younger children.[12,13] Children 1-5 years of age tend to have more severe dengue cases.[14] Seventy percent of mortality was reported in children <15 years with the highest CFR at age 0-4.[15] This is because the younger the child's age, the less developed the physiological and anatomical functions of the child. The presence of microvascular differences at a younger age is more prone to plasma leakage compared with older age.[16] In addition, the immune system that is not well-developed cause healing time to become much longer. The duration of immune responses in fighting infection is longer in children.[17]
Patients with DSS were mostly women with short duration of hospitalization. In addition, clinical fluid accumulation such as pleura effusions was commonly found in DSS patient. Low platelet also shown in this study. Patients with DSS presented more frequently bleeding manifestations, elevated liver enzyme, and change in hematocrit leve.l[18-20] Regarding the hemodynamic outcome, the results showed that most children had an increased HR. The average HR was higher in aged group 1-2 years old. An increase in heart frequency values occurring higher in DSS patients compared with dengue patients without shock in children.[18] The respiratory rate also increases in the state of shock as a compensation of the decrease in oxygen in tissues resulting in anaerobic product accumulation resulting in PH changes that may cause metabolic acidosis. The average RR was higher in group under 1-year-old and decreasing by age.
The average blood pressure, PP, and MAP were lower in children under 2 years old. lowest systolic pressure occurred in children age group of 35 years (70 mmHg) while PP below 20 mmHg was found in aged between 1-2 years old. This occurs in the defervescence phase (toxic stage) of DSS where plasma leakage occurs causing decrease of CO and results in shock conditions.[21] In Vietnam, as many as 36% of children have a decrease in left ventricular systolic function with a <50% ejection fraction.[22] In India, as many as 16.7% of children have ejection fraction values <50%.[23] The occurrence of cardiac dysfunction may be caused by direct damage from DENV, the host's own hostile response to the virus, decreased myocardial contractility or coronary hypoperfusion due to decreased CO.[21,24-26]
Meanwhile, MAP signifies an indicator of arterial pressure of organ perfusion.[27] MAP is associated with CO. This association was demonstrated by a study of adults with DSS found that Cardiac Output declined significantly on day 6 in proportion to MAP values. The value of MAP decreased at days 4-6 (critical phase) of 74-76 mmHg.[18] In this study, the lowest number of Mean Arterial Pressure was found in 1-5 years old children with 57 mmHg. However, most children had normal MAP values. The value of MAP is used in regulating the rate and amount of intravenous fluids that should be given to the patient.[28] Normal MAP is possible because the patient has been treated with fluids before being referred to Dr. Soetomo General Hospital. Therapy with Hypertonic saline solutions (HSSs) 3% and 7.5% can rapidly restore MAP versus 250mL of Ringer Lactate within an hour.[29] Therefore, all parameters need to be considered as a support in determining the hemodynamic conditions of the DSS patient.
Despite the WHO guidelines for identifying DSS, shock manifestations and the classification of DHF / DSS may differ in different populations with different ethnicities. Many literatures suggest that the general characteristics of DSS patients do not necessarily meet criteria by WHO.[30,31] In some literature it is found that the patient may be in a state of shock but does not describe a decrease in blood pressure or a significant increase in heart rate.[32] Therefore, the results obtained in everyday clinical life do not always follow the actual theory. It is necessary for the doctors to make an accuracy early hemodynamic monitoring to provide appropriate therapy for the patients.
This study has several limitations. First, the researchers only describe hemodynamic outcome
based on clinical parameters without a tool. Second, this study cannot describe the cause of mortality in patients specifically. Thirdly, this study was conducted in a single hospital, which may limit its generalizability of the data.
CONCLUSION
The hemodynamic outcome varies on each group based on age. Disease incidence of DSS cases remained the highest in older children but mortality rates were the highest in younger children. Predictors of mortality from DSS were not identified in this study.
References
-
1. World Health Organization. Dengue and Severe
Dengue,
http://www.who.int/mediacentre/factsheets/fs1
17/en/; 2016 [accessed 12 February 2017].
-
2. World Health Organization. CD Risk Assessment; DF, DBD and DSS in Indonesia, http://www.who.int/diseasecontrol_emergencie s/guidelines/Dengue_ind_risk%20assess.pdf;
2005 [accessed 12 February 2017].
-
3. Departemen Kesehatan RI. Kemenkes Terima Laporan Peningkatan Kasus DBD di Jawa Timur, http://www.depkes.go.id/article/view/15013000 002/kemenkes-terima-laporan-peningkatan-kasus-dbd-di-jawa-
timur.html#sthash.k81F01M4.dpuf; 2015
[accessed 12 February 2017].
-
4. Raihan, Hadinegoro SRS, Tumbelaka AR.
Faktor Prognosis Terjadinya Syok pada Demam Berdarah Dengue. Sari Pediatr. 2010;12(1):47–52.
-
5. Dejnirattisai, W., Jumnainsong, A., Onsirisakul, N., dkk. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328(5979):745-8.
-
6. Srichaikul T, Nimmannitya S. Hematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol. 2000 Jun;13(2):261-76.
-
7. Kementrian Kesehatan RI. Situasi DBD. Jakarta Selatan: Pusat Data dan Informasi; 2016.
-
8. Pongpan, S., Wisitwong, A., Tawichasri, C., Patumanond, J., & Namwongprom, S.
Development of Dengue Infection Severity Score. ISRN Pediatrics. 2013; 1–6.
-
9. Coffey, L. L., Mertens, E., Brehin, A.-C., Fernandez-Garcia, M. D., Amara, A., Després, P., & Sakuntabhai, A. Human genetic
determinants of dengue virus susceptibility. Microbes Infect. 2009;11(2);143–56.
-
10. Tantracheewathorn, T., Tantracheewathorn, S. Risk Factors of Dengue Shock Syndrome in Children. J Med Assoc Thai. 2007;90(2):272-7.
-
11. Guzman, MG., Alvarez, M., Halstead, SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibodydependent enhancement of infection. Arch Virol. 2013;158(7):1445-59.
-
12. Limkittikul , K., Brett, J., L'Azou, M.
Epidemiological Trends of Dengue Disease in Thailand (2000–2011): A Systematic Literature Review. PLoS Negl Trop Dis.
2004;8(11):e3241.
-
13. Kamath SR, Ranjit S. Clinical features, complications and atypical manifestations of children with severe forms of dengue hemorrhagic fever in South India. Indian J Pediatr. 2006; 73:889-95.
-
14. Tantawichien, T. Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr Int Child Health. 32 Suppl 2012;1:22-7. doi:
10.1179/2046904712Z.00000000049.Bethell, DB., Gamble, J., Loc, PP., dkk. Noninvasive Measurement of Microvascular Leakage in Patients with Dengue Hemorrhagic Fever. Clin Infect Dis. 2001;32(2):243-253.
-
15. Hanna-Wakim R, Yasukawa LL, Sung P, dkk. Age-related increase in the frequency of CD4(+) T cells that produce interferon-gamma in response to staphylococcal enterotoxin B during childhood. J Infect Dis. 2009 Dec 15;200(12):1921-7.
-
16. Thanachartwet V, Wattanathum A,
sahassananda D., dkk. Dynamic Measurement of Hemodynamic Parameters and Cardiac Preload in Adults with Dengue: A Prospective Observational Study. Plos One. 2016 May 19;11(5):e0156135.
-
17. Lovera D, Martinez de Cuellar C, Araya S, dkk. Clinical characteristics of Dengue Shock Syndrome (DSS) in children. Pediatr Infect Dis J. 2016 Dec;35(12):1294-1299.
-
18. Wali JP, Biswas A, Chandra S, dkk. Cardiac involvement in Dengue Haemorrhagic Fever. Int J Cardiol. 1998;64(1):31–6. [PubMed]
-
19. Khongphatthanayothin, A., Lertsapcharoen, P., Supachokchaiwattana, P., La-Orkhun,
V., Khumtonvong, A., Boonlarptaveechoke, C., Pancharoen, C. Myocardial depression in dengue hemorrhagic fever: Prevalence and clinical description. Pediatr Crit Care Med.2007;8(6):524-9.
-
20. Yacoub, S., Wertheim, H., Simmons, CP., Screaton, G., Wills, B. Cardiovascular
Manifestations of The Emerging Dengue
Pandemic. Nat Rev Cardio. 2014;11: 335-345.
-
21. Kabra SK, Juneja R, Madhulika, Jain Y, Singhal T, Dar L, Kothari SS, Broor S. Myocardial
dysfunction in children with dengue haemorrhagic fever. Natl Med J India. 1998 Mar-Apr;11(2):59-61.
-
22. Miranda, CH., Borges, Mde C., Matsuno, AK., Vilar, FC., Gali, LG. Evaluation of Cardiac Involvement During Dengue Viral Infection. Clin Infect Dis.2013;57(6):812-9.
-
23. A. Khongphatthallayothin, P.
Chotivitayatarakorn, S. Somchit, A. Mitprasart, S. Sakolsattayadorn, C. Thisyakorn. Morbitz type I second degree AV block during recovery from dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2000 Dec;31(4):642-5.
-
24. Wichmann D, Kularatne S, Ehrhardt S, Wijesinghe S, Brattig NW, Abel W, Burchard GD. Cardiac involvement in dengue virus in dengue virus infections during the 2004/2005 dengue fever season in Sri Lanka. Southeast Asian J Trop Med Public Health. 2009 Jul;40(4):727-30.
-
25. Dünser, MW., Takala, J., Ulmer, H., dkk. Arterial blood pressure during early sepsis and
outcome. Intensive Care Med.
2009;35(7):1225-33.
-
26. Pizarro-Torres D. Dengue With Severe Plasma Leakage: A New Monitoring Approach. Acta Méd Costarric. 2016; 58(3): 115-121.
-
27. Han J, Ren HQ, Zhao QB, Wu YL, Qiao ZY. Comparison of 3% and 7.5% Hypertonic Saline in Resuscitation After Traumatic Hypovolemic Shock. Shock. 2015 Mar;43(3):244-9.
-
28. Balmaseda, A., Hammond, SN., Pérez, MA., dkk. Short report: assessment of the World Health Organization scheme for classification of dengue severity in Nicaragua. Am J Trop Med Hyg. 2005;73(6):1059-62.
-
29. Cao, XT., Ngo, TN., Wills, B., dkk. Evaluation of the World Health Organization standard tourniquet test and a modified tourniquet test in the diagnosis of dengue infection in Viet Nam. Trop Med Int Health. 2002;7(2):125-32.
-
30. Opreanu RC, Arrangoiz R, Stevens P, Morrison CA, Mosher BD, Kepros JP. Hematocrit,
systolic blood pressure and heart rate are not accurate predictors for surgery to control hemorrhage in injured patients. Am Surg. 2010 Mar;76(3):296-301.
-
31. Victorino GP, Battistella FD, Wisner DH. Does tachycardia correlate with hypotension after trauma?. J Am Coll Surg. 2003 May;196(5):679-84.
16
Discussion and feedback