TRISPECIFIC BROADLY NEUTRALIZING ANTIBODIES, N6/PGDM1400-10E8V4 DELIVERED BY RECOMBINANT ADENO-ASSOCIATED VIRUS (RAAV8) VECTOR: A NOVEL VACCINE FOR HIV/AIDS INFECTION
on
ARTIKEL TINJAUAN PUSTAKA

ESSENTIAL:Essence of Scientific Medical Journal (2019), Volume 17, Number 1:25-33
P-ISSN.1979-0147, E-ISSN. 2655-6472
TINJAUAN PUSTAKA
TRISPECIFIC BROADLY NEUTRALIZING ANTIBODIES, N6/PGDM1400-10E8V4 DELIVERED BY RECOMBINANT ADENO-ASSOCIATED VIRUS (RAAV8) VECTOR: A NOVEL VACCINE FOR HIV/AIDS INFECTION
Ghea Mangkuliguna,1a Glenardi,1
ABSTRAK
Latar Belakang: HIV/AIDS telah menjadi masalah kesehatan global yang menyebabkan kematian pada jutaan orang setiap tahunnya. Kebutuhan akan suatu terapi yang efektif telah menjadi sebuah urgensi global yang harus segera direalisasikan. Namun sayangnya, keberagaman dari amplop glikoprotein pada HIV telah menjadi tantangan terbesar dalam mengembangkan terapi HIV yang efektif. Belakangan, sebuah inovasi terapi terbaru bernama broadly neutralizing antibodies (bnAbs) telah berhasil dikembangkan dan diperkirakan dapat menjadi solusi nyata bagi para penderita HIV/AIDS. Namun untuk dapat diterapkan secara klinis, bnAbs perlu dikombinasikan bersama bnAbs lainnya agar dapat menangani diversitas dari amplop glikoprotein HIV. Baru-baru ini, peneliti telah berhasil mengombinasikan tiga jenis bnAbs yang berbeda ke dalam sebuah molekul yang diberi nama sebagai trispesifik N6/PGDM1400-10E8v4. Trispesifik ini mampu menargetkan tiga epitope utama HIV secara bersamaan dan menjadi terapi yang paling efektif hingga saat ini dalam menetralkan berbagai strain dari HIV.
Pembahasan: Trispesifik N6/PGDM1400-10E8v4 terbukti mampu menetralkan hingga 99% dari 208 strain HIV yang diujicoba hanya dengan dosis IC50<1,0 μg/ml. Hal ini dapat dicapai karena trispesifik mampu menargetkan epitope HIV yang berbeda secara bersamaan tanpa mengurangi afinitas antibodi induknya. Selain itu, rAAV8 yang digunakan sebagai media penghantar bagi trispesifik juga berperan dalam menjaga ekspresi dan sekresi dari bnAbs agar dapat bertahan dalam jangka waktu yang panjang.
Simpulan: Dengan demikian, dapat disimpulkan bahwa trispesifik N6/PGDM1400-10E8v4 memiliki potensi yang menjanjikan untuk menjadi vaksin HIV/AIDS.
Kata Kunci: broadly neutralizing antibodies, HIV, rAAV8, trispesifik N6/PGDM1400-10E8v4
ABSTRACT
Introduction: HIV/AIDS has already become one of the world's major health issues taking its toll on millions of lives each year. Developing an HIV vaccine with excellent efficacy has become a global urgency that must be addressed immediately. However, the highly diversified HIV-1 envelope glycoprotein (Env) proved to be a challenge in generating an effective HIV vaccine and therapy. Broadly neutralizing antibodies (bnAbs) as the latest therapy shows excellent promise as a novel vaccine to fight the HIV epidemic. Nevertheless, bnAb as an individual therapeutic agent is not potent enough to fight against the diversity of the HIV epitope. Recently, trispecific N6/PGDM1400-10E8v4, a combination of three various bnAbs has been developed and it has been proven to be the most potent therapy developed at present.
Discussion: This literature review yield results that trispecific N6/PGDM1400-10E8v4 was able to neutralize a large margin of 208 HIV-1 strains up to 99% at IC50<1.0 μg/ml by targeting multiple epitopes at the same time without losing each of its parental antibodies affinity. We particularly highlight the use of rAAV8 to deliver the bnAbs in order to ensure long-term expression and secretion of antibodies in those infected with HIV.
Conclusion: Thus, the potent and breadth neutralizing ability of trispecific N6/PGDM1400-10E8v4, making it an excellent candidate to become the future HIV vaccine.
Keyword: broadly neutralizing antibodies, HIV, rAAV8, trispecific N6/PGDM1400-10E8v4
1Undergraduate Medical Program, School of Medicine and Health Sciences, Atma Jaya Catholic University of Indonesia
aEmail: mangkuligunaVG14 02@gmail.com
INTRODUCTION
Human immunodeficiency virus (HIV) is a dangerous virus that attacks the humans’ immune system, especially the CD4 cells. This virus specifically attacks the CD4 immune cells and completely destroys them. Without adequate treatments, HIV can further develop to acquired immunodeficiency syndrome or AIDS, a condition in which the immune system is no longer capable of fulfilling its role in fighting infection and disease. This condition is known as the opportunistic infection - a phenomena in which HIV/AIDS-infected individuals are very susceptible to infections.[1]
According to World Health Organization (WHO) and Joint United Nations Programme on HIV/AIDS (UNAIDS), since the beginning of the AIDS epidemic, around 76.1 million people have been infected with the virus and 39 million people have passed away
from it.[2] There is no effective therapy that can cure this disease. The medicine that had been used for decades to treat HIV/AIDS is called antiretroviral therapy (ART). It does not cure HIV infection, but only maintain HIV to undetectable levels.[1,3] Moreover, there are only 30% of people infected with HIV that successfully suppressed HIV to undetectable levels.[2] As a result, such high rates of AIDS-related deaths continue to occur globally. ART as the current HIV therapy is necessary but not competent enough to end the global HIV/AIDS epidemic. In the early years of the HIV/AIDS epidemic, immunotherapy against HIV-1 was developed. Human monoclonal antibodies (mAb) appear to be a new therapy for HIV/AIDS patients. Treatment with mAb showed a slight improvement compared with other therapies that have been used
since the AIDS epidemic. However, mAbs also had limited breadth against multiple HIV-1 strains.[4]
Several years after the finding of mAB, researchers have tried to transfer antibodies from the sera of HIV-1-infected subjects to AIDS patients. Unfortunately, result suggested that these antibodies were ineffective against the diverse and highly adaptive virus. However, these studies demonstrated that a small fraction of HIV-1+ patients could produce antibodies that target shared epitopes across various clades of HIV-1. These finding also encourages researchers to develop the next generation of HIV-1 mAbs.[4]
Recently, a new method has been developed from the Nussenzweig laboratory. This method allows direct cloning and sequencing heavy and light chain genes of immunoglobulin (Ig) directly from B cells. These findings led to the rapid identification of multiple mAbs with much greater potency and breadth than the previous antibodies. The new antibodies that have greater potency and breadth in neutralizing is known as broadly neutralizing antibodies (bnAbs).[1]
However, bnAbs as individual therapeutic agents were not potent enough for practical use. The improvements in both potency and breadth are needed to become a promising therapy for HIV-1 infection. There are few ways to improve the potency and breadth of a bnAb. One of them is achieved by combining multiple bnAbs into a single molecule. Recently, several multispecific bnAbs has been developed, but the most recent one that shows promise is the trispecific bnAbs.[3,5] There are various combination of trispecific bnAbs, but the most powerful one is N6/PGDM1400-10E8v4. Consisted of three different types of bnAbs makes trispecific N6/PGDM1400-10E8v4 the most broad-spectrum multispecific bnAbs at this time. [6]
N6, one of the trispecific constituents, is a CD4-binding site antibody. It has just recently discovered in 2016 and has been proven to have greater neutralizing ability compared to other CD4-binding site antibodies. The next trispecific constituents is PGDM1400. It inhibits the binding of V1/V2 glycans into α4β7 integrin receptor in human body cells. PGDM1400 is the latest and strongest V1/V2 glycan antibody. Other bnAb that composes trispecific is 10E8v4, a gp41 membrane proximal extracellular region (MPER) antibody. As a second generation of MPER antibody, 10E8v4 is the most effective antibody in its group. It can neutralize HIV isolates with a dose that is 2 times lower than other bnAbs in this group.[5]
The combination of these three powerful bnAbs makes the trispecific N6/PGDM1400-10E8v4 not only the most broad-spectrum trispecific bnAbs, but also the most powerful in neutralizing HIV isolates. In this study, we reported the effectivity and neutralizing capability of trispecific N6/PGDM1400-10E8v4 in multiple HIV strains.[3,5]
DISCUSSION
Broadly neutralizing antibodies (bnAbs) to engage human immunodeficiency virus (HIV) infection
Broadly neutralizing antibodies (bnAbs) are antibodies capable of neutralizing the majority of strains of a given highly antigenically variable pathogen.[5] In the case of HIV, it can target shared epitopes found across the virus subtypes. bnAbs
could be found naturally among 10-50% untreated HIV-infected individual. An even smaller percentage individual develops more effective types of bnAbs. These leads to discovery of bnAbs types with more potency and larger breadth that can cover many of the virus subtypes. However, the body’s natural response to create bnAbs take years prior to infection. On the other hand, after several years, the naturally produced bnAbs are not adequate as the individual has already been exposed to large variants of HIV strains.[3]
Advances in technology allow for bnAbs usage as a promising prophylactics and therapeutic agent. Various trials have succeeded in identifying and isolating potent bnAbs in untreated HIV-infected individual. These potent bnAbs could be further manufactured to an effective and manageable amount. Thus, through intermittent blood transfusion, these potent bnAbs can be administered to other people in need[3,5] As a vaccine, bnAbs can be administered to pre-exposed individuals to induce early protection against the virus[6] As for therapy, bnAbs can be combined with antiretroviral treatment (ART) for remission and eradication of HIV-virus.[6]
bnAbs works by targeting proteins expressed on the HIV-1 envelope such as the critical CD4 binding site; glycan-coated viral loops, including the V1/V2 glycan and V3 glycan, gp120-gp21 interface and a conserved viral membrane-proximal external region (MPER).[1] CD4 binding site (CD4bs) antibodies types block conformational epitope on the trimeric Env glycoprotein. As CD4bs is crucial for the viral entry, blockage at the receptor will inactivate the virus. The first ever identified CD4bs antibody is VRC01. A study by Ledgerwood et al in 2015 has reported results of first clinical trial testing VRC01 as prophylactics in humans. The results show potentially protective serum up to 8 weeks after second infusion and has proven the possibility of CD4bs antibody as vaccine.[2,4] The development continues as several other CD4 binding site antibodies such as VRC07, b12, and 3BNC117 have been found to have greater coverage and potency. At the latest, the most effective antibody of the CD4bs type is N6. N6 shows extraordinary breadth and potency than any other CD4bs antibodies as it target more conserved epitopes in various panels of virus. [10]
Examples of V3 Glycan-Dependent Antibodies are PGT121, KD-247, 10-1074. Structural studies have shown that PGT121 interferes CD4 binding to gp120 despite showing no interaction with the CD4 binding site. It instead interferes by allosteric mechanism involving V3 base, N332 oligomannose glycan, and surrounding glycans which locked into conformation that block CD4 binding sites. Other notable types of bnAbs includes CAP256 which is a V2-Glycan Dependent Antibodies and bnAb 108e which target MPER.
Several trials have been conducted to further explore bnAbs capabilities. Studies by Xu et al and Julg et al demonstrated complete protection of rhesus macaques from a swarm of SHIV variants by a bnAb mixture of PGT121 (targets the V3 glycan) and PGDM1400 (targets the V1/V2 glycan) -separate usage failed to do so.[1] Nishimura et al have also conducted a study that shows passive immunotherapy with bnAb cocktails could prevent mother to child transmission, suppress viremia, and facilitate CD8+ T-cell immunity for durable suppression of virus in non-human primates.[2] A
study evaluates a combination of bnAbs comprising 3BNC117, 10–1074 and PG16 in humanized mice infected with the HIV‑ 1YU2 isolate. Infusion of this bnAb cocktail after infection resulted in decreased viremia in ~50% of the mice.[4] All the trials show positive results in bnAbs’ potential as a prophylactics and a therapeutic agent. Although further exploration is necessary as humans are exposed to diverse HIV strain "swarm".
In spite of showing novel promises, prophylactics and therapeutic use of bnAbs still face challenges. Usage of single-target bnAbs has shown rebound viremia from outgrowth low-frequency resistant in the post-treatment of several non-human primates or person. One example is the infusion of 10 mg/kg PGT121 that shows viral rebound in 42-56 days. These impose a problem for single bnAbs usage in long-term treatment.[5,6] In conclusion, there is an urgency in developing bnAbs to achieve more potency, larger breadth, and greater efficacy.
Combination of N6, PGDM1400, and 10E8v4 in a single molecule targeting multiple epitopes of HIV Env yields greatest neutralization potency
One of the most promising targets of the bnAbs is the CD4bs in gp120 of HIV envelope. For HIV infection to occur, it has to bind to CD4, one of the receptors found in the surface of the host cell.[7] Recently discovered in 2016, N6 is a highly broad
and potent antibody that belongs to the class that binds CD4bs. A study conducted by Huang et al revealed that N6 has the ability to neutralize 181 cross-clade and 171 clades C pseudoviruses up to 96% and 98% respectively with a median IC50 lower than 0.1 μg/ml. (see Table 1) Its remarkable potency and breadth are marked by its unique recognizing of the CD4 binding site.[8,9] While most CD4-class antibodies show considerable sensitivity to strains 93TH057 and DU172 however, N6 was proven to have a higher binding affinity to the gp120 proteins.[8,10] Moreover, N6 could strongly bind to gp120 protein from strain X2088, one of the most highly resistant strain to almost all CD4bs antibodies,[10] as well as CD4bs mutants (e.g. gp120 D368R and gp120 resurfaced stabilized core 3 (RSC3) D371I-P363N) of which the other antibodies belonging to the same class could not do.[8] Compared with other VRC01-class antibodies, N6 appeared to have an altered binding angle relative to CD4 (5-8 degrees), 0.5 Å smaller translation distance and rotated light-chain N-terminus.[8,9] This evidence suggested that N6 slightly moved away from the V5 region of gp120 indicating its ability to evade steric clash, a mechanism associated with the HIV resistance to CD4bs antibodies.[9] These characteristics of N6 makes it possible to interact with HIV envelope of which cannot be done by other VRC01-class antibodies while at the same time achieving remarkable breadth and potency.
Table 1. Neutralization potency and breadth of N6, PGDM1400, and 10E8 comparison to other bnAbs, against a 181-isolate Env-pseudovirus panel.[8]
|
N6 |
7RC27 VRCOt |
3BNC 117 |
PG9 |
PGDM 1400 |
PGT 121 |
10 1074 |
10E8 |
4E10 |
35022 | ||
|
No. Ofviruses |
181 |
175 |
177 |
181 |
177 |
171 |
177 |
178 |
180 |
181 |
181 |
|
iCsι<50 μg∕ml |
98% |
78% |
89% |
83% |
78% |
78% |
64% |
66% |
98% |
98% |
62% |
|
ICw <1 μg∕ml |
96% |
56% |
73% |
76% |
64% |
70% |
50% |
60% |
72% |
37% |
48% |
|
GM ‰+ |
0.044 |
0.297 |
0.250 |
0.094 |
0.109 |
0.015 |
0.051 |
0.036 |
0.222 |
1.303 |
0.058 |
|
Median ICw |
0.038 |
0.217 |
0.248 |
0.073 |
0.088 |
0.008 |
0.022 |
0.022 |
0.352 |
1.920 |
0.033 |
Binctingsite CD4-binding site V1V2 V3 gp41 MPER 9p1
gp4i
Geometric Mean ICm concentration is μg∕ml
The V1/V2 glycan site is one of the most important epitope in HIV envelope. It marks the beginning of HIV invasion to the host cell. V1/V2 glycan site will bind into α4β7 Integrin Receptor in host cell and start the binding of HIV into the host cell. The inhibition of V1/V2 glycan site binding to α4β7 Integrin Receptor will make the HIV unable to invade the host cell, thus HIV infection will never occur and the person will have an absolute protection. There are several V1/V2 glycan site antibodies has been developed, but PGDM1400 is the recent one.[2] The fragment antigen binding (Fab) of the PGDM1400 determined by the 34-residue CDRH3 that have an extended β-hairpin conformation similar to the CDRH3 of PGT145. It binds to the trimer apex via its
CDRH3 arm. A triad of aspartic acid residues in the tip of CDRH3 provides a highly anionic potential to interact with cationic residues in the gp120 V1/V2. PGDM1400 exhibited exceptional breadth and potent neutralization activity.[11,12] According to research conducted by Huang et al, it is markedly superior to PGT151, PGT121, and PGT128, which are the most potent bnAbs existed to date. PGDM1400 was also capable of neutralizing 78% of the virus from 170 viruses studied. (see Table 1)
Another promising target for HIV therapy is the membrane-proximal external region (MPER) in gp41 subunit of HIV Env. Recently, the second-generation of anti-MPER bnAb, 10E8, was discovered and found to be the broadest known
neutralizing antibodies against HIV-1. 10E8 targets not only a helical epitope in the membrane-proximal external region (MPER), but also the transmembrane domain (TMD) in gp41.[13] It has an exceptional ability to attain specific and high-affinity binding to the MPER at the viral membrane interface, thus increasing its neutralizing activity and becomes the most effective anti-MPER antibody. This is indicated by 10E8 ability to neutralize 98% of a panel of 180 diverse HIV-1 isolates as shown in Table 1.[14] Despite its great ability, 10E8 has a low solubility that
impedes its delivery in the human body. However, by combining the mutational alteration of hydrophobic surface patches and the incorporation of natural variation from somatic variants of the 10E8 lineage, researchers have succeeded in engineering a 10E8 variant with high solubility. (see Figure 1) The 10E8v4 as the new variant of 10E8 has the potency and breadth nearly indistinguishable from the 10E8. It has higher bioavailability and longer half-life than the parent 10E8.[13,14,15]
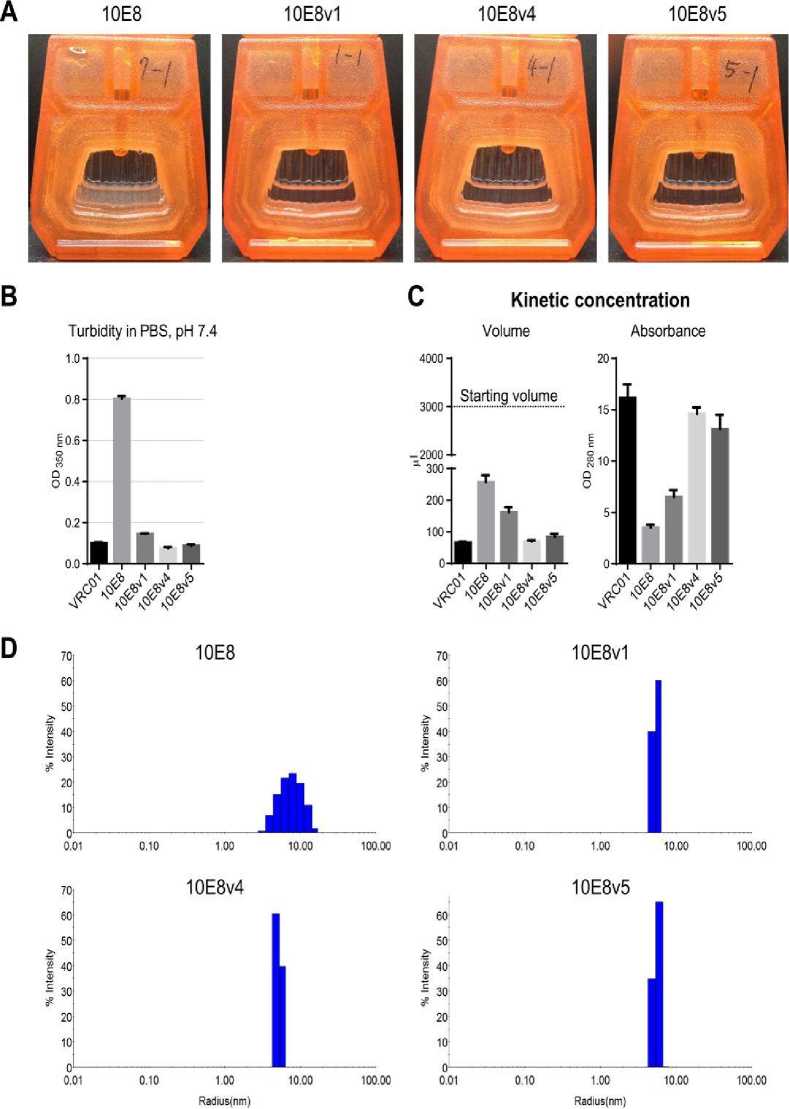
Figure 1. 10E8v1, 10E8v4, and 10E8v5 are soluble and monodisperse.[15]
Trispecific N6/PGDM1400-10E8v4 as a promising broad and potent neutralizing antibodies as a novel vaccine for HIV infection
As described above, whether it is N6, PGDM1400, or 10E8v4, each of them possessed exceptional ability in neutralizing various HIV strains. Recent studies revealed that the incorporation of
multiple specific broadly neutralizing antibodies in a single molecule would yield much greater efficacy in protecting or treating HIV infection.[6,16]
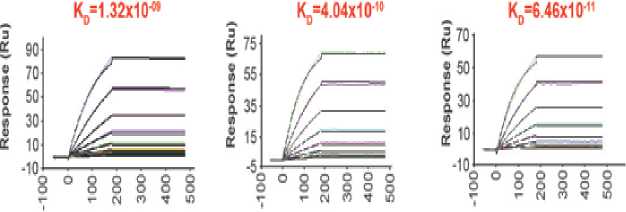
In a study conducted by Xu et al, trispecific N6/PGDM1400-10E8v4 is designed using knob-in-hole heterodimerization making up the single arm (N6) and cross-over dual variable Ig-like proteins (CODV-Ig) to generate the double arms (PGDM1400-10E8v4) as shown in Figure 2. The main feature found in the double arms is the circular self-contained linkage serving as a self-supporting
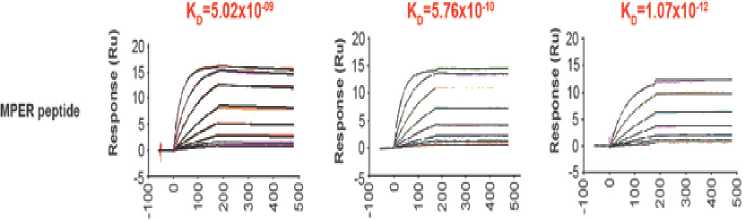
framework.[16,17] This novel molecular modeling strategy enables trispecific N6/PGDM1400-10E8v4 to target multiple epitopes at the same time without losing each of its parental antibodies' affinity. Evidence suggesting that trispecific N6/PGDM1400-10E8v4 is able to sequentially bind to each parental antibodies target's epitope is shown in Figure 3. The equilibrium binding constant (Kd) of trispecific N6/PGDM1400-10E8v4 was in fact on par with the affinity of parental antibodies.
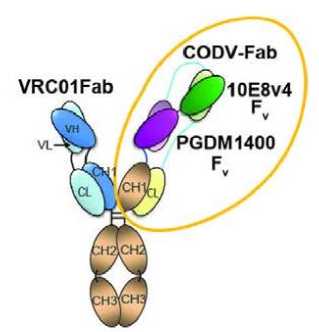
Figure 2. Crystal structure of the CODV Fab and a structural model of the trispecific N6/PGDM1400-10E8v4. Note that N6 is represented by VRC01 as both antibodies belong to the same class.[16]
PGDM1400 VRC01∕PGDM1400-10E8v4 ΔVRC ∕PGDM1400- IOE8v4
Ko=5.90x10* Kb=IJOxIO* Kb=1.77x10*
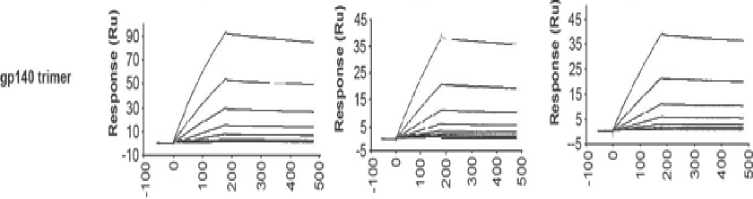
Time (Sec.) Time (Sec.) Time(Sec1)
1OE8v4 VRC01∕PGDM1400-10E8v4 ΔVRC01∕ΔPGDM148 ∙10E8v4
Time (Sec.∣ Time (Sec.) Time (Sec.)
VRC01 VRC01fPGDM1400-10E8v4 VRC01∕ΔPGDM1400-Δ10E8v4
Time (Sec.) Time (Sec.) Time (Sec.)
Figure 3. The binding affinity of individual components in the trispecific Ab in comparison to parental bnAbs. Note that N6 is represented by VRC01 as both antibodies belong to the same class.[16]
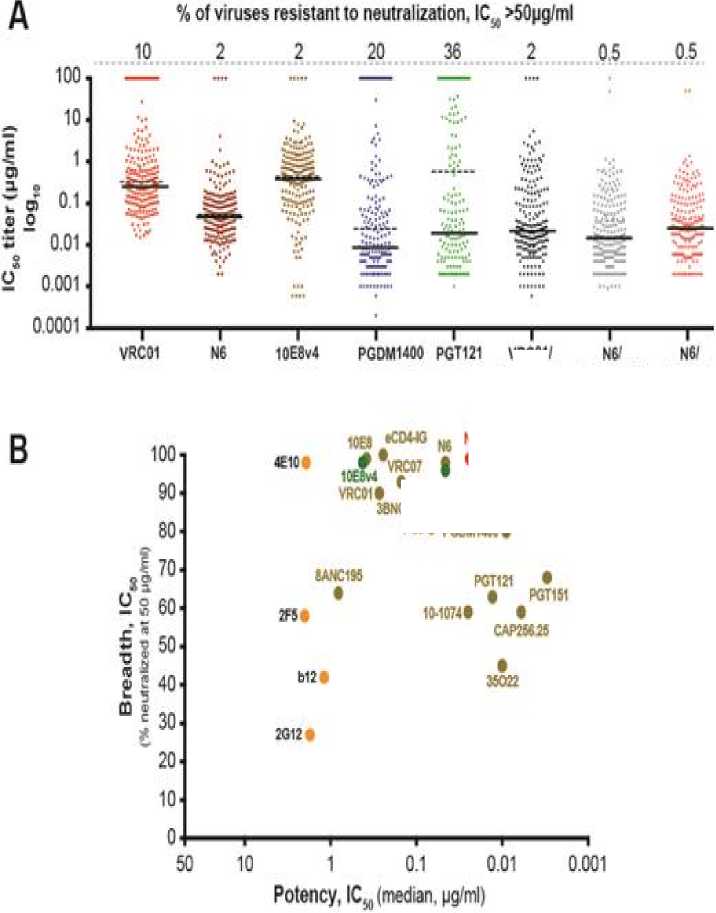
The same study also revealed that trispecific N6/PGDM1400-10E8v4 was able to neutralize a large margin of 208 HIV-1 strains up to 99% at IC50 lower than 1.0 μg/ml (see Table 2). In accordance with Figure 4 as well, the best neutralization potency and breadth is well-observed in trispecific N6/PDGM1400-10E8v4. Both of the overall median IC50 neutralization titer of sensitive virus (solid line)
and 208 HIV strains (dotted line) in trispecific N6/PGDM1400-10E8v4 are less 0.02 μg/ml.[16] This discovery is yet to be tested in humans' clinical trials. However, in vivo study suggested that trispecific N6/PGDM1400-10E8v4 shows promise as the most potent and broad multispecific antibodies that has ever been created.
Table 2. Overall breadth and potency of trispecific Abs compared to parental bnAbs and eCD4-Ig against a representative panel of 208 HIV-1 strains.[16]
∙CD4
PGDM
10EBv4
10E8v4
cdm 10E8v4
PGDM
1400
PGDM
PGTWI PGT121
^GOMiwmv* "fJLPGDM-.
1* ) H 1400 NNHN 1400
PGDM
1400
|
%IC50 <50ug∕ml |
90 |
98 |
100 |
98 |
80 |
63 |
98 |
99 |
95 |
99 |
99 |
99 |
|
%IC50<1.0ug∕ml |
74 |
96 |
85 |
73 |
75 |
52 |
90 |
99 |
87 |
98 |
85 |
98 |
|
Median IC50 (sensitive) |
0.271 |
0.046 |
0.244 |
0.422 |
0.009 |
0.013 |
0.022 |
0.015 |
0.013 |
0.024 |
0.041 |
0.018 |
|
Geometric mean |
0.280 |
0.046 |
0.129 |
0.309 |
0.017 |
0.024 |
0.034 |
0.021 |
0.025 |
0.028 |
0.075 |
0.020 |
|
Median IC 50 W |
0.329 |
0.046 |
0.244 |
0.437 |
0.025 |
0.579 |
0.022 |
0.015 |
0.015 |
0.026 |
0.041 |
0.018 |
|
Geometric mean |
0.462 |
0.053 |
0.164 |
0.349 |
0.086 |
0.403 |
0.039 |
0.021 |
0.036 |
0.030 |
0.080 |
0.022 |
Delivering trispecific N6/PGDM1400-10E8v4 as broadly neutralizing HIV antibodies using recombinant adeno-associated virus (rAAV8) vector
Based on the above facts and figures, trispecific N6/PGDM1400-10E8v4 shows promise in eliminating HIV Infection. According to Brady et al, a single intravenous injection of bnAb every three weeks gives a temporary reduced viral rebound in phase I clinical trials.[17] These studies prompted the repeated administration of bnAb which would render lower patient's compliance. In addition, the cost of manufacture, purification, and quality control on a large scale of antibodies would be too exaggerating.[18,19] Such approach in passive transfer of bnAb is deemed inefficient and thus an alternative is needed to express biologically active antibodies in a prolonged manner without having to administer bnAbs several times. In this case, recombinant adeno-associated virus (rAAV) vector appears to be a major breakthrough of this problem.
Adeno-associated virus (AAV) is a small (20 nm), non-enveloped virus. It belongs to genus Dependoparvovirus, which in turn belonging to family Parvoviridae. It is a replication-defective virus, meaning that it depends on the assistance of a coinfecting helper virus.[18] In these recent years, the application of AAV vector-mediated gene transfer has gained a lot of attention as it is able to mediate desired-protein for the lifetime of the cell as long as the product is considered as self by one's immune
system.[19] AAV can genetically modify a lot of dividing and non-dividing cells with a minimal pathologic response.[20]
Requiring a single dose of administration, AAV particles also show high resistance to high temperature and widely-range pH, as well as stability after being stored for a long time. Despite the given benefits of AAV, it has a limited capacity of 4.4-5 kb in packaging single-stranded DNA genome.[18,21] A rAAV vector only structurally consists of desired-gene flanked by cis-acting AAV inverted terminal repeat (ITR) sequences necessary for replication and packaging. Trans-acting Rep and Cap protein play an important role as helper proteins for the replication-defective rAAV in cells.[18] Previous studies yield result in the sustained expression of the intramuscularly-injected transgene using rAAV vector. Noted that trispecific antibodies would need two heavy-chain and light-chain arms in order to bind to multiple HIV epitopes.[21] Thus, at least two different vectors are needed to effectively deliver the antibodies without losing each of their affinity to target antigens.
Here, we proposed the use of rAAV8 to efficiently deliver trispecific N6/PGDM1400-10E8v4 antibodies both as a prevention and treatment for HIV infection. Several studies have shown the potential of rAAV8 in expressing both heavy and light chains by incorporating the 2A self-processing peptide.[18] This novel space-saving strategy is a major development in handling the limited capacity of
rAAV at the same time has remarkable antigenbinding activity and prolonged expression in vivo. Next, we will discuss this matter from immunogenicity context. As shown in a study done by Boutin et al, 72% of the human race had pre-existing immunity to AAV2, 67% to AAV1, while 38% to AAV8.[19,21] This number is two times lower than that of AAV2. Other serotypes such as AAV 3, 5, and 6 has somewhat less transduction efficacy while AAV 1 and 4 showed
no activity at all.[20] It is also important to note that natural AAV infection has been associated with neither serious symptoms nor diseases.[18] Therefore, in this review, we highlight the use of rAAV8 to achieve a long-term transgene delivery in humans’ bodies, particularly in those infected with HIV. The delivery of trispecific N6/PGDM1400-10E8v4 antibodies is boosted to a point where its effectivity reaches beyond average.
IOEM-PGDM∣4M
Figure 4. Neutralization titers of trispecific antibodies and broadly neutralizing antibodies, and sequential binding of alternative Env epitopes.[16]
VRCOIi
PGDM1<OO- PGDM1400∙
UEM IOEM
N⅛10EM√>GOM1C0
I ∙ NftPGDMICfriOCM e ,^.^WOH^msM • VRC07-523
κs∙ PG0Mf4X∙
Evaluating the use of trispecific N6/PGDM1400-10E8v4 instead of bispecific or monospecific neutralizing HIV antibodies
One of the problem in single target bnAbs is the development of resistant mutation in HIV strains that can lead to viral rebound. Therefore, there is a need to target multiple epitopes at a time to further increase the neutralization capacity of the vaccine. Xu et al have developed methods of fusing two or three epitopes binding into a single molecule known as bispecific or trispecific bnAbs. Bi-tri specific bnAbs offer great alternatives in targeting multiple
envelopes, which reduce the occurrence of resistant mutations due to its greater potency and breadth.[6]
Studies have compared the effectiveness among single, bi and trispecific bnAbs in terms of potency and breadth.[6] One study found that tri-NAb neutralization potency was greater than individual parental bnAbs or the Bi-NAb (Figure 6), with an outstanding IC50 geometric mean of 0.069 μg/ mL, which is at least four-fold lower than that of the VRC01,10E8,and Bi -NAbdVRC01-5X-PGT121.
A recent study in 2017 identified the two most effective combination candidates of trispecific antibodies which is VRC01 / PGDM1400-10E8v4 and N6/PGDM1400-10E8v4. When tested on 208
virus panel, PGDM1400-10E8v4 showed large potency and breadth, however, 4 resistant viruses were still found. On the other hand, N6/PGDM1400-10E8v4 left only 1 resistant virus. N6/PGDM1400-10E8v4 has also been compared to one of the most potent monospecific bnAbs, N6. It was found that N6
is 5-fold less potent than the N6/PGDM1400-10E8v4 trispecific Ab. N6 targets only a single epitope, which could increase the chance of viral escape mutations explained before.[16] In conclusion, the combination of N6/PGDM1400-10E8v4 was deemed to be the most potent.
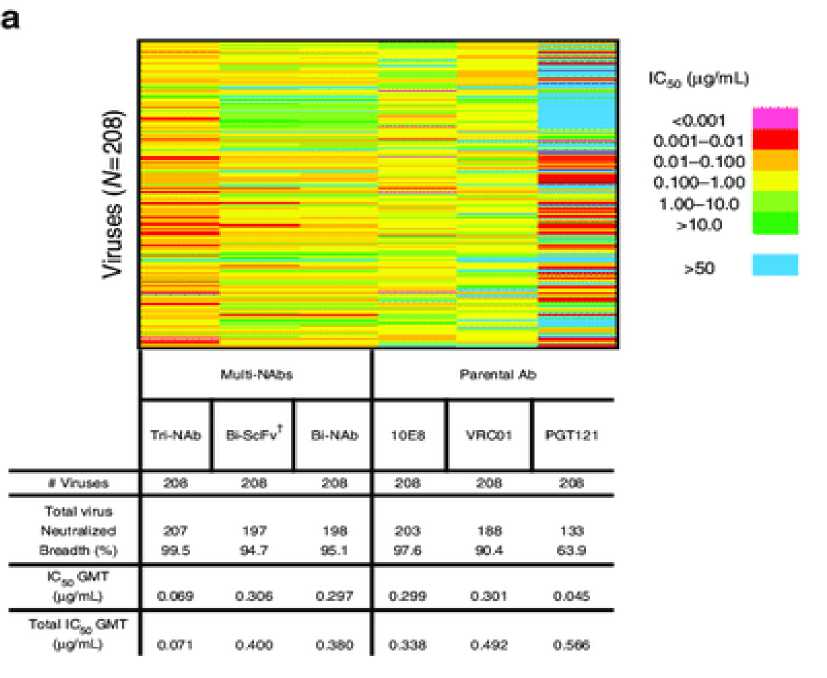
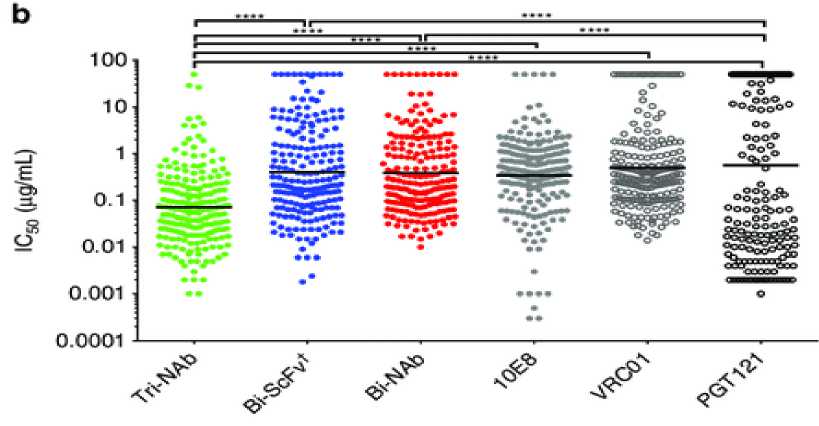
Figure 5. (a) Neutralization breadth of the parental, bispecific and trispecific antibodies was tested against a panel of 208 viral strains. (b) Scatter plots of IC50 titers in which each virus is represented by an individual dot.[6]
CONCLUSION
The extensive genetic diversity in HIV has been a challenge to develop an effective HIV vaccine. In the last few years, the next generation of human monoclonal antibodies called broadly neutralizing antibodies (bnAbs) have been developed as a new solution for the HIV epidemic.
However, bnAbs as individual therapeutic agents are not potent enough to fight the diversity of the HIV epitope. Recently, trispecific N6/PGDM1400-10E8v4, a combination of three various bnAbs has been developed and it has been proven to be the most potent therapy developed at present. Trispecific N6/PGDM1400-10E8v4 neutralize a large margin of
208 HIV-1 strains up to 99% at a very little concentration. It also targets multiple HIV epitopes at the same time without losing each of its parental antibodies affinity. Furthermore, the use of rAAV8 to deliver trispecific N6/PGDM1400-10E8v4 has been proven to improve its effectiveness and also achieved a long-term antibody expression in HIV-infected patients. Thus, the results suggest that trispecific N6/PGDM1400-10E8v4 appears to have the potential to be an effective vaccine to prevent HIV infection in the near future.
REFERENCES
-
1. Gama L, Koup RA. New-generation high-potency and designer antibodies: role in HIV-1 treatment. Annu Rev Med. 2018;69:14.1-14.11.
-
2. Stephenson KE, Barouch DH. Broadly neutralizing antibodies for HIV eradication. Springer. 2016;13:31–7.
-
3. Burton DR, Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol. 2016;34:635–59.
-
4. Ferrari G, Haynes BF, Koenig S, Nordstorm JL, Margolis DM, Tomaras GD. Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. Springer Nat. 2016;15.
-
5. Cohen MS, Corey L. Broadly neutralizing antibodies to prevent HIV-1. AAAS. 2017;358(6359).
-
6. Steinhardt JJ, Guenaga J, Turner HL, McKee K, Louder MK, O’Dell S, et al. Rational design of a trispecific antibody targeting the HIV-1 Env with elevated anti-viral activity. Nat Commun. 2018 Feb 28;9(1):877.
-
7. Wang H, Chen X, Wang D, Yao C, Wang Q, Xie J, et al. Epitope-focused immunogens against the CD4-binding site of HIV-1 envelope protein induce neutralizing antibodies against auto- and heterologous viruses. J Biol Chem. 2018;293(3):830–46.
-
8. Huang J, Kang BH., Ishida E, Zhou T, Griesman T, Sheng Z, et al. Identification of a CD4-binding-site antibody to HIV that evolved nearpan neutralization breadth. Elsevier Inc. 2016;45:1108–21.
-
9. Julg B, Pegu A, Abbink P, Liu J, Brinkman A, Molloy K, et al. Virological control by the CD4-binding site antibody N6 in SHIV-infected rhesus monkeys. Journal of Virology. 2017;91(16).
-
10. Sok D, Burton DR. HIV broadly neutralizing antibodies: taking good care of the 98%. Elsevier Inc. 2016;45.
-
11. Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer
protection against a clade C SHIV challenge. Science Translational Medicine. 2017;9.
-
12. Sok D, Pauthner M, Julian J-P, Saye-Francisco KL, Hsueh J, Briney B, et al. Recombinant HIV envelope trimer selects for quaternarydependent antibodies targeting the trimer apex. PNAS. 2014;111(49):17624–9.
-
13. Rujas E, Leaman DP, Insauti S, Ortigosa-Pascual L, Zhang L, Zwick MB, et al. Functional optimization of broadly neutralizing HIV-1 antibody 10E8 by promoting membrane interactions. JVI. 2018;92:e02249-17.
-
14. Wagh K, Seaman MS, Zingg M, Fitzsimons T, Barouch DH, Burton DR, et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLOS Pathogens.
2018;14(3):e1006860.
-
15. Kwon YD, Georgiev IS, Ofek G, Zhang B, Asokan M, Bailer RT, et al. Optimization of the Solubility of HIV-1-Neutralizing Antibody 10E8 through Somatic Variation and Structure-Based Design. JVI. 2016;90(13):5899–914.
-
16. Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358(6359):85–9.
-
17. Steinmetz A, Beil C, Lange C, Baurin N, Beninga J, Corvey C, et al. CODV-Ig, a universal bispecific tetravalent and
multifunctional immunoglobulin format for medical applications. Taylor & Francis Group. 2016;8(5):867–78.
-
18. Brady JM, Baltimore D, Balazs AB. Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. John Wiley Sons Ltd. 2017;275:324–33.
-
19. Fuchs SP, Desrosiers RC. Promise and
problems associated with the use of recombinant AAV for the delivery of anti-HIV antibodies. Mol Ther Methods Clin Dev. 2016;3:16068.
-
20. Hemphill DD, McIlwraith CW, Goodrich LR. Adeno-associated viral vectors show serotype specific transduction of equine joint tissue explants and cultured monolayers. Sci Rep. 2014;4:5861.
-
21. Gardner MR, Farzan M. Engineering antibodylike inhibitors to prevent and treat HIV-1 infection. Wolters Kluwer Health Inc. 2017;12(3):294–301.
33
Discussion and feedback