PRODUCTION OF BIODIESEL FROM WASTE COOKING OIL BY TRANSESTERIFICATION REACTION USING CaO/NATURAL ZEOLITE CATALYSTS
on
Cakra Kimia (Indonesian E-Journal of Applied Chemistry)
Volume 5, Nomor 2, Oktober 2017
PRODUCTION OF BIODIESEL FROM WASTE COOKING OIL BY TRANSESTERIFICATION REACTION USING CaO/NATURAL ZEOLITE CATALYSTS
I Made Wisnu Adhi Putra
Faculty of Health, Science, and Technology, University of Dhyana Pura, Bali Indonesia wisnuadhiputra@undhirabali.ac.id
ABSTRAK: Biodisel adalah metil ester asam lemak yang dihasilkan melalui reaksi transesterifikasi antara minyak dan lemak dengan alkohol dalam kehadiran katalis. Dalam penelitian ini, pembuatan biodisel dari minyak jelantah dan metanol melalui reaksi transesterifikasi telah berhasil dilakukan menggunakan katalis CaO/zeolit alam. Metode impregnasi basah dipilih untuk menyintesis katalis dengan cara mengaduk campuran zeolit alam dengan CaO dalam sistem refluks pada 90 oC selama 3 jam. Jumlah CaO terimpregnasi pada zeolit alam adalah 5%wt, 10%wt, dan 15%wt. Karakterisasi katalis menggunakan FTIR, XRD, dan N2 sorption analyzer menunjukkan bahwa katalis dengan 15%wt CaO yang terimpregnasi merupakan katalis yang paling baik. Reaksi transesterifikasi dilakukan dalam variasi rasio molar minyak:metanol, waktu reaksi, dan jumlah katalis. Analisis menggunakan GC-MS menunjukkan bahwa kandungan biodisel didominasi oleh metil palmitat, dan metil oleat. Konversi biodisel tertinggi ditemukan pada kondisi rasio minyak:metanol yaitu 1:15, waktu reaksi 5 jam, dan katalis 5%wt.
Kata kunci: biodisel, transesterifikasi, minyak jelantah, CaO, zeolit.
ABSTRACT: Biodiesel is fatty acid methyl esters (FAME) produced by transesterification reaction of oils and fats with an alcohol in the presence of a catalyst. In this work, production of biodiesel from waste cooking oil and methanol by transesterification reaction has been successfully conducted using CaO/natural zeolite catalysts. Wet impregnation method was choosen to synthesize the catalyst by stirring the mixture of natural zeolites and CaO in reflux apparatus at 90 oC for 3 hours. The amount of CaO impregnated into natural zeolite were 5%wt, 10%wt, and 15%wt. The catalysts characterization using FTIR, XRD, and N2 sorption analyzer showed that catalyst with 15%wt CaO impregnated into zeolites was the greatest one. Transesterification reaction was carried out in various oil to methanol molar ratio, reaction time, and amount of catalyst. GC-MS analysis confirmed that the biodiesel contents were dominated by methyl palmitate and methyl oleate. The highest biodiesel conversion was found under condition of 1:15 oil to methanol molar ratio, 5 hours of reaction time, and 5%wt of catalyst.
Keywords: biodiesel, transesterification, waste cooking oil, CaO, zeolites.
The energy crisis issue has triggered many of researchers in the world to intensively search alternative fuels and can be used to replace fossil fuels. In recent years, the production of biodiesel as
alternative fuel has occurred very fast [1]. Biodiesel is fatty acid methyl esters (FAME) produced from the transesterification reaction of oils and fats with an alcohol in the presence of a catalyst. It is non-toxic, biodegradable, and
free of sulfur or carcinogenic compounds. With those properties, biodiesel becomes the first choice to replace petrodiesel [2].
Transesterification reaction may occurred in the presence of an acid or a base catalyst, but a base catalyst was found to accelerate the reaction faster than an acid catalyst and it was also need less attention. Therefore, the production of biodiesel from oil or fat catalyzed by base was more studied than that by acid. Homogenous base catalyst was the most frequently used in transesterification reaction. According to the research conducted by Mirie et al. [3], biodiesel yielded by transesterification of cotton seed oil using NaOH and KOH catalysts were 68.60% and 83.94% respectively.
Despite the use of homogeneous base catalysts give a high yield at low temperature, but it remains some weaknesses such as difficulty in catalyst separation from the system, generation of large amounts of waste water, reactor corrosion, and the catalyst cannot be used again after the reaction [4]. Another difficulty in the use of homogeneous basic catalysts is due to the sensitivity of the free fatty acid and water to produce the phenomenon of saponification [5]. These weaknesses can be overcome with the use of heterogeneous base catalyst. This catalyst has the advantages of being easily separated from the system and can be reused [6]. Another advantage of the use of heterogeneous base catalyst is the reaction does not produce the phenomenon of saponification, glycerol purification becomes easier, and more tolerant of free fatty acids from raw materials [7].
Calcium Oxide (CaO) is the most often heterogeneous base catalyst often used in the transesterification reaction because it has properties of high alkalinity, low cost, non-corrosive, low solubility and easy to prepare from widely available materials of natural origin or waste precursors [8]. In addition, CaO catalyst is also easily separated from the product and it is not toxic. Calcium Oxide shown the excellent
potential as a catalyst of transesterification reaction, but the biodiesel percentage yielded was still low [9, 10]. One reason might be caused by CaO was large in size, so that the surface area of the catalyst was relatively small and the particles were not well distributed. Some researchers have conducted research in order to enlarge the surface area of the CaO catalyst. Among several methods, the use of support materials as a host of CaO has been widely investigated. Albuquerque et al. [11] used CaO supported on mesoporous silicas (SBA-15, MCM-41 and fumed silica) as catalysts in transesterification of vegetables oil. The results showed that the biodiesel conversion was as high as 95% for sunflower oil (5 hours) and 65% (1 hour) for castor oil. Other results about the use of CaO supported on various host materials in transesterification reaction were also reported [12, 13].
This research was aimed to produce biodiesel from waste cooking oil using CaO/natural zeolite catalysts. The catalyst was made by inserting CaO into the natural zeolite pores using impregnation method. Natural zeolites are alumina-silicate polycrystalline known to have pore size in the nanometer scale, making it suitable as a host material of CaO. The parameters tested in this study were the effect of waste cooking oil to methanol molar ratio, mass of catalyst, reaction time and reaction temperature.
Natural zeolites were obtained from subdistrict of Bayah, Banten, Indonesia. Waste cooking oil was from food merchant around of Tabanan, Bali, Indonesia. Calcium oxide, ammonium chloride, methanol, sodium hydroxide, and hydrochloric acid were purchased from Merck. All of purchased reagents were analytical grade.
-
2.2 Instrumentation
Instruments used in this study were: three-necked glass reactor, reflux apparatus, magnetic stirrer, glassware, pH meter, 200 mesh siever, muffle furnace, desiccator, bulb suction, titration instrument, filter paper, analytical scale, stative, condenser, and thermometer. Philips analytical diffractometer, Shimadzu 8201 FTIR spectrophotometer, Quantacrome N2 sorption analyzer were used to characterize the catalysts. Gas Chromatography-Mass spectrophotometer (Shimadzu GCMS-QP2010) was used to characterize the biodiesel yield.
Natural zeolites used in this study were 200 meshes sized. This material was soaked in distilled water while stirring and then dried in an oven at a temperature of 120 °C for 2 hours. The dried natural zeolites were then refluxed with addition of NH4Cl 1 M at a temperature of 90 °C for 8 hours. Once completed, the mixture was filtered and washed with distilled water until pH = 6 and dried in an oven at a temperature of 130 oC. The solid was crushed and placed in a porcelain dish and calcined for 4 hours at a temperature of 500 °C in a muffle furnace. Activated natural zeolites were analyzed using FTIR, XRD, and N2 sorption analyzer.
Synthesis of the catalysts
A 50 g of activated natural zeolites and 100 ml of CaCl2 5 %wt were refluxed at 90 °C for 3 hours. The mixture was then evaporated while stirring in order to obtain the solid form. Subsequently, the mixture was dried at a temperature of 130 °C for 2 hours and crushed. The dried catalyst was calcined at a temperature of 500 °C for 3 hours. This synthesized catalyst was notified as CaO/NZ5% catalysts. The same procedure was also performed for other types of catalysts, namely: CaO/NZ10% and CaO/NZ15%. The catalysts was further
characterized by FTIR, XRD and N2 sorption analyzer.
Transesterification Process
Transesterification reaction of waste cooking oil was conducted following the procedure stated by Endalew et al. [14]. The molar ratio of waste cooking oil to methanol was 1:15. The amount of catalyst was 5%wt (Based on the amount of waste cooking oil) and the stirring speed was 300 rpm. Methanol and catalyst were placed in three-necked glass reactor. The mixture was stirred and heated by using stirring hotplate until it reached the temperature of 60 oC. Separately, 100 g of waste cooking oil was heated up to 60 oC and poured into the mixture of methanol and catalyst. The final mixture was then refluxed for 7 hours. The reaction result was centrifuged at 5700 rpm to obtain clear sparation. The upper phase was collected and evaporated to remove methanol phase. GC-MS was used to qualitatively measure the biodiesel content. Biodiesel yield was calculated using the formula below [15]:
Massof Biodieselx%FAME BiodieselYield = x100%
Massof Oil
Where % FAME shows the concentration of Fatty Acid Methyl Ester obtained by GC analysis. The same prosedure was also conducted for the variation of molar rasio of waste cooking oil to methanol (1:6, 1:9, and 1:15),
reaction time (1, 3, 5, 7, and 9 hours), and mass of catalyst (1, 3, 5, 7, and 9 g).
Fourier Transform Infrared
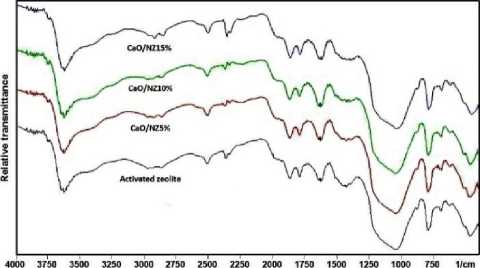
Spectroscopy spectra of the CaO/natural zeolite are shown in Figure. 1a. Note that there are no significant alteration on absorption peaks. All of the CaO/natural zeolite catalysts show the same characteristic with the activated zeolite. This result indicates that the structure of
natural zeolite was not changed when CaO impregnated into natural zeolites. Figure 1b exhibits the XRD pattern of CaO/natural zeolite catalysts. All of the catalysts have the same pattern with natural zeolite even after calcination indicates that CaO does not alter the mineral structure of natural zeolite. In this research, zeolite will restrict the growth of CaO crystals and causing the
CaO particle size become smaller, so that increasing the surface area of CaO, as has been postulated by Misriyani et al. [16]. It can be explained that most of CaO have highly dispersed on the surface of natural zeolite to form monolayer interaction. Thus, the CaO phase did not appear in the pattern [17].
(a)
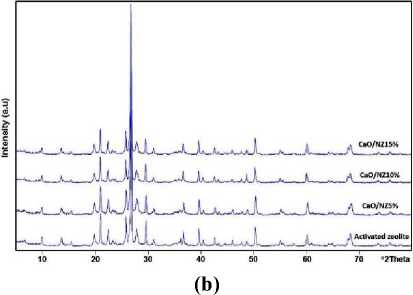
Figure 1. Characterization of the catalysts: (a) FTIR spectra, (b) XRD pattern
|
Table 1. Effect of The Amount of CaO on Surface Area of Catalysts | ||
|
No |
Variable [unit] |
Surface Area [m2/g] |
|
1 |
Natural zeolite |
35.076 |
|
2 |
Activated zeolite |
51.445 |
|
3 |
CaO/NZ5% |
15.201 |
|
4 |
CaO/NZ10% |
15.326 |
|
5 |
CaO/NZ15% |
12.928 |
Table 1 summarizes the results of the surface area of the catalysts. It can be seen that the surface area of the catalysts decrease as the amount of CaO impregnated into natural zeolite increase. There are two reasons that can explain this phenomenon. First, it is caused by partial blocking of the zeolite network by CaO particles. Second, it is caused by the filling of zeolite pores by CaO particles [11, 17]. Thus, the larger the amount of CaO impregnated into natural zeolites, the lower the surface area of the catalyst. Moreover, the pores of zeolite were occupied by CaO and dispersed in a particular portion. This resulted the decrease of specific surface area of zeolite. Based on the data showed in
Table 1, CaO/NZ15% catalyst was choosen for transesterification reaction of waste cooking oil.
Figure 2 shows that the higher the amount of methanol, the higher the biodiesel percentage. The highest result was achieved at 1:15 molar ratio. Further, biodiesel percentage decreased when oil to methanol molar ratio increased beyond 1:15. This result was caused by the solubility of glycerol as by product of the reaction increased. As a consequence, glycerol was arduously separated from the mixture. The high content of glycerol in the mixture can shift back the reaction equilibrium to the reactant portion [18]. Viriya-empikul et al. [19] reported that the reverse transesterification reaction occurs between the biodiesel product and glycerol. This reaction resulted monoglycerides and diglycerides that could homogenize the products because its co-solvent behavior.
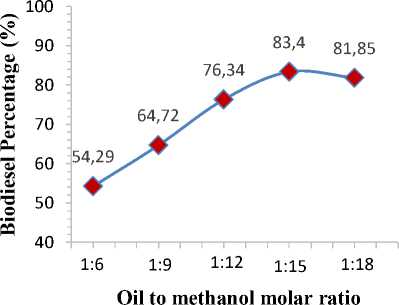
Figure 2. Effect of waste cooking oil to methanol molar ratio on biodiesel percentage
In this study, the transesterification reaction was carried out at different time from 1-9 hours. Whereas oil to methanol molar ratio, mass of catalyst and reaction time was maintained at 1:15, 5 g, and 3 hours, respectively. The result is shown in Figure 3.
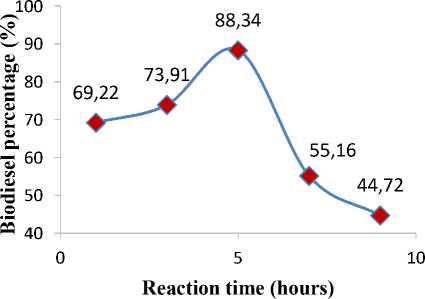
Figure 3. Effect of reaction time on biodiesel percentage
According to the result shown in Figure 3, it can be seen that the biodiesel percentage increases with the reaction time increases. The highest yield (88.34%) was achieved in 5 hours. Subsequently, biodiesel yield was decreased steadily beyond 5 hours due to increasing production of glycerol. Since the transesterification reaction was reversible, excess glycerol may shifted back the
equilibrium to the left. Thus, the production of biodiesel decreased drastically [20, 21].
The effect of catalysts mass was investigated to find the optimum amount of catalyst in biodiesel production. For this purpose, mass of catalyst was varied at 1-7 g. Other variables such as oil to methanol molar ratio, reaction time, and reaction temperature were kept constant according to the previously works. Effect of mass of catalyst to biodiesel conversion is depicted in Figure 4. It can be seen that the biodiesel conversion raises as the amount of catalyst increases. The highest biodiesel conversion was attained at 5 g of catalyst. Further increment of biodiesel conversion was not found when excess catalysts were introduced. Beyond 5 g, the biodiesel conversion was decreased drastically. It might be caused by the increase of mixture viscosity giving a serious stirring problem. Thus, the more powerful stirring was needed in order to have high biodiesel conversion [22]. In addition, Roschat et al. [23] stated that the large amount of catalyst loading (beyond 6%wt) would never give a high yield of biodiesel because of mass transfer of reactants to the catalyst was limited, where the mass transfer was the determining step of the reaction.
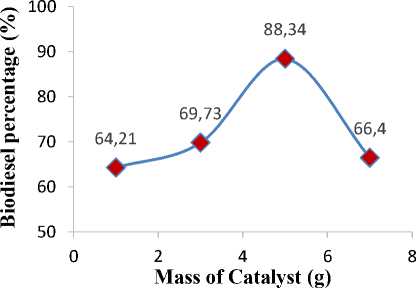
Figure 4. Effect of catalyst mass on biodiesel percentage
Gas Chromatography-Mass spectro-
photometer (Shimadzu GCMS-QP2010) was used to characterize the biodiesel yield. The result of biodiesel analysis using GC-MS is shown in Table 2. Based on the GC-MS analysis, biodiesel content was dominated by methyl palmitate and methyl oleate with retention time of 16.407 and 18.124 min respectively.
Table 2. Quantitative Results of Biodiesel
|
Name |
R. Time |
m/z |
Area |
Height |
|
Hexadecano ic acid, methyl ester |
16.407 |
74.0 |
126429 |
72050 |
|
9-Octadece noic acid (Z)-, methyl ester |
18.124 |
55.0 |
42919 |
20899 |
CaO/natural zeolite catalysts has been successfully synthesized using wet impregnation method. The FTIR and XRD results showed that CaO particles impregnated into natural zeolite did not affect the structure of zeolite. The surface area of the catalysts measured using N2 sorption analyzer was found decreasing as the amount of CaO impregnated into zeolite increasing from 15.201 m2/g to 12.928 m2/g. Transesterification reaction of waste cooking oil yielded the highest biodiesel conversion under condition of 1:15 oil to methanol molar ratio, 5 hours reaction time, and 5%wt of catalyst.
Author would like to thank to Research and Community Service, Dhyana Pura Institute for the internal research funding.
-
[1] Sawangkeaw R., Tejvirat P., Ngamcharassrivichai C. and
Ngamprasertsith S. Supercritical transesterification of palm oil and hydratedethanol in a fixed bed reactor
with a CaO/Al2O3 catalyst. Energies. 2012, 5, 1062–1080.
-
[2] Vuppaladadiyam A.K., Sangeetha C.J. and Sowmya V.
Transesterification of
pongamiapinnata oil using base catalysts a laboratory scale study. Univers. J. Environ. Res. Technol. 2013, 3, 113–118.
-
[3] Mirie S.N., Thiongo G.T., Kioni P.N., Kariuki P.N. and Namaru W.S. Optimization of biodiesel production from cotton seed oil using KOH and NaOH as catalysts, Scientific Conference Proceedings. 2010, 285– 293.
-
[4] Stojković I.J., Stamenković O.S., Povrenović D.S. and Veljković V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renew. Sust. Energy Rev. 2014, 32, 1–15.
-
[5] Nakagaki S., Bail A., dos Santos V.C., de Souza V.H.R., Vrubel H., Nunes F.S., and Ramos L.P. Use of anhydrous sodium molybdate as an efficient heterogeneous catalyst for soybean oil methanolysis. Appl. Catal. A. 2008, 351, 267–274.
-
[6] Chouhan A.P.S. and Sarma A.K., Modern heterogeneous catalysts for biodiesel production: a
comprehensive review. Renew. Sust. Energ. Rev. 2011, 15, 4378–4399.
-
[7] Gopal S. and Sajitha C.M. Production of biodiesel from vegetable oil using CaO catalyst and analysis of its performance in four stroke diesel engine. IJSER. 2013, 3, 1–6.
-
[8] Kostić M.D., Bazargan A., Stamenković O.S., Veljković V.B. and McKay G. Optimization and kinetics of sunflower oil methanolysis catalyzed by calcium oxide-based catalyst derived from palm kernel shell biochar. Fuel. 2016, 163, 304– 313.
-
[9] Reddy C., Reddy V., Oshel R. and Verkade J.G. Room-temperature conversion of soybean oil and poultry
fat to biodiesel catalyzed by nanocrystalline calcium oxides. Energy & Fuels. 2006, 20, 1310–
1314.
-
[10] Putri E. M. M., Rachimoellah M., Santoso N. and Pradana F. Biodiesel production from kapok seed oil (ceibapentandra) through the
transesterification process by using CaO as catalyst. GJRE. 2012, 12.
-
[11] Albuquerque M.C.G., Jiménez-Urbistondo I., Santamaría-González J., et al. CaO supported on mesoporoussilicas as basic catalysts for transesterification reactions. Appl. Catal. A. 2008, 334, 35–43.
-
[12] Witoon T., Bumrungsalee S., Vathavanichkul P., Palitsakun S., Saisriyoot M., and Faungnawakij K. Biodiesel production from
transesterification of palm oil with methanol over CaO supported on bimodal meso-macroporous silica catalyst. Bioresour. Technol. 2014,
156 329–334.
-
[13] Jia L., Li Y., Chen J., Guo X., S. Lou, and Duan H. Montmorillonite-supported KF/CaO: a new solid base catalyst for biodiesel production. Res. Chem. Intermed. 2015, 42, 1791–
1807.
-
[14] Endalew A. K., Kiros Y. and Zanzi R. Heterogeneous catalysis for biodiesel production from Jatrophacurcas oil (JCO). Energy. 2011, 36, 2693–2700.
-
[15] Bet-Moushoul E., Farhadi K., Mansourpanah Y., Nikbakht A.M., Molaei R. and Forough M. Application of CaO-Based Au nanoparticles as heterogeneous nanocatalysts in biodiesel production. Fuel. 2015, 164, 119–127.
-
[16] Misriyani, Kunarti E.S. and Yasuda M. Synthesis of Mn(II)-Loaded TixSi1-XO4 Composite Acting as a Visible-Light Driven Photocatalyst. Indones. J. Chem., 2015, 15(1), 4349.
-
[17] Wu H., Zhang J., Wei Q., Zheng J. and Zhang J. Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts. Fuel Process. Technol. 2013, 109, 13–18.
-
[18] Srivastava A. and Prasad R. Triglycerides-based diesel fuels. Renew. Sust. Energ. Rev. 2000, 4, 111-133.
-
[19] Viriya-empikul N., Krasae P., Nualpaeng W., Yoosuk B. and Faungnawakij K. Biodiesel
production over Ca-based solid
catalysts derived from industrial
wastes. Fuel. 2012, 92, 239–244.
-
[20] Shan R., Chen G., Yan B., Shi J. and Liu C. Porous Cao-based Catalyst derived from PSS-induced
mineralization for biodiesel
production enhancement. Energ.
Convers. Manage. 2015, 106, 405– 413.
-
[21] Chen G., Shan R., Yan B., Shi J., Li S. and Liu C. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process. Technol. 2016, 143, 110–117.
-
[22] Tang Y., Chen G., Zhang J. and Lu Y. Highly active CaO for the transesterification to biodiesel production from rapeseed oil. Bull. Chem. Soc. Ethiop. 2011, 25, 37–42.
-
[23] Roschat W., Siritanon T., Yoosuk B. and Promarak V. Biodiesel production from palm oil using hydrated lime-derived CaO as a lowcost basic heterogeneous catalyst. Energ. Convers. Manage. 2016, 108, 459–467.
57
Discussion and feedback