THE EFFECT OF NEEM GUM ON THE REDUCTION IN RAT BLOOD GLUCOSE LEVELS INDUCED BY STREPTOZOTOCIN
on
Buletin Veteriner Udayana Volume 15 No. 5: 982-990
pISSN: 2085-2495; eISSN: 2477-2712 Oktober 2023
Online pada: http://ojs.unud.ac.id/index.php/buletinvet https://doi.org/10.24843/bulvet.2023.v15.i05.p35
Terakreditasi Nasional Sinta 4, berdasarkan Keputusan Direktur Jenderal Pendidikan Tinggi, Riset, dan Teknologi No. 158/E/KPT/2021
The Effect of Neem Gum on the Reduction in Rat Blood Glucose Levels Induced by Streptozotocin
(EFEK NEEM GUM TERHADAP KADAR GL UKOSA DARAH TIKUS YANG DIINDUKSI STREPTOZOTOSIN)
Ichlasul Mahdi Fardhani1, Jauhar Firdaus2*, Zahrah Febianti3, Hairrudin3, Cholis Abrori4, Elly Nurus Sakinah4
-
1Faculty of Medicine, University of Jember, Jember, East Java, Indonesia, 68121;
-
2Department of Physiology, Faculty of Medicine, University of Jember, Jember, East Java, Indonesia, 68121;
-
3Department of Biochemistry, Faculty of Medicine, University of Jember, Jember, East Java, Indonesia, 68121;
-
4Department of Pharmacology, Faculty of Medicine, University of Jember, Jember, East Java, Indonesia, 68121;
*Corresponding author email: jauhar_firdaus. fk@unej.ac.id
Abstract
Pharmacological therapy for diabetes, which is primarily made up of chemicals, still causes a lot of side effects. The numerous side effects of pharmacological therapy for diabetes have generated innovations in alternative diabetes therapies using ingredients derived from nature. One of the natural ingredients with an anti-hyperglycemia effect is neem gum (NG), or the sap of the neem plant (Azadirachta indica). The aim of this study was to determine the effect of NG on the reduction of blood glucose levels. Moreover, this study had six groups, including a normal control group and groups with doses of 0, 3.75, 7.5, 15, and 30 grams/kgBW. The results showed that NG reduced blood glucose levels in diabetic rats. The paired t-test showed a significant reduction in blood glucose in all groups administered NG except for the dose 3.75 group (P<0.05). The minimum and maximum effective doses of NG were calculated using the quadratic regression test with the equation y = 1.059x2–46.576x+408. With a target blood glucose level of between 80 and 126 mg/dL, the minimum and maximum effective doses of NG were obtained in the range of 12–15 mg/dL. This study concluded that administering NG to diabetic rats can reduce blood glucose levels. Phytochemical studies and research are needed with serial examinations using the effective dose range of neem gum.
Keywords: Azadirachta indica; diabetes mellitus; hyperglycemia; neem gum
Abstrak
Terapi farmakologi untuk diabetes, yang terutama terdiri dari bahan kimia, masih menimbulkan banyak efek samping. Banyaknya efek samping terapi farmakologis untuk diabetes telah melahirkan inovasi terapi alternatif diabetes dengan menggunakan bahan-bahan yang berasal dari alam. Salah satu bahan alami yang memiliki efek antihiperglikemia adalah getah nimba (NG), atau getah tanaman nimba (Azadirachta indica). Tujuan dari penelitian ini adalah untuk mengetahui pengaruh NG terhadap penurunan kadar glukosa darah. Selain itu, penelitian ini memiliki enam kelompok, yaitu kelompok kontrol normal dan kelompok dengan dosis 0, 3,75, 7,5, 15, dan 30 gram/kgBB. Hasil penelitian menunjukkan bahwa NG menurunkan kadar glukosa darah pada tikus diabetes. Uji-t berpasangan menunjukkan penurunan glukosa darah yang signifikan pada semua kelompok yang diberikan NG kecuali untuk kelompok dosis 3,75 (P<0,05). Dosis efektif minimum dan maksimum NG dihitung menggunakan uji regresi kuadrat dengan persamaan y = 1,059x2–46,576x+408. Dengan target kadar glukosa darah antara 80 dan 126 mg/dL, dosis efektif minimum dan maksimum NG diperoleh dalam kisaran 12-15 mg/dL. Penelitian ini menyimpulkan bahwa pemberian NG pada tikus diabetes dapat menurunkan kadar glukosa darah. Diperlukan adanya studi fitokimia dan penelitian dengan pemeriksaan serial dengan menggunakan rentang dosis efektif neem gum.
Kata kunci: Azadirachta indica; diabetes mellitus; hyperglycemia; neem gum
INTRODUCTION
Diabetes mellitus (DM) is a group of metabolic diseases characterized by hyperglycemia that develop as a result of abnormalities in insulin secretion, insulin action, or both (Indonesian Society of Endocrinology (PERKENI), 2021). According to the International Diabetes Federation (2021), the global prevalence of diabetes among people aged 20 to 79 years has reached 537 million. Meanwhile, Indonesia ranked seventh among the ten countries with 10.7 million diabetics. This number is predicted to increase further, and Indonesia will be ranked fourth globally in terms of the number of DM patients (Ministry of Health of the Republic of Indonesia, 2020; World Health Organization, 2018). The main symptom of DM is a rise in blood glucose, known as hyperglycemia. Hyperglycemia is a risk factor for a variety of diabetic complications, including damage to the eyes, kidneys, nerves, heart, and peripheral vascular system. If complications develop, the patient will be considerably more difficult to treat because the patient’s disease will be irreversible at this stage (Elgebaly et al., 2019).
Administration of anti-diabetic drugs (OAD) as pharmacological therapy to control the glycemic status of DM patients, such as sulfonylureas, metformin, thiazolidinedione, insulin, and others, still has numerous side effects that may impact the patient’s quality of life. Some of the side effects include gastrointestinal, metabolic, hypoglycemia, weight gain, and others (Hameed et al., 2022; PERKENI, 2021; Putra et al., 2017). Because of the numerous negative effects associated with the use of OAD, the majority of which are made up of chemicals, it is essential to conduct research on innovations using natural ingredients for glycemic control. One of the natural ingredients with an antihyperglycemia effect is neem gum (Azadirachta indica), or the sap of the neem plant (Malviya et al., 2017; Bashar et al., 2021).
Polysaccharides found in neem gum or the sap of the neem plant includes d-galactose, l-arabinose, d-glucuronic acid, and d-xylose. These polysaccharides are speculated to be responsible for the antihyperglycemic activity because they are non-digestible polysaccharides. Furthermore, Wiyono et al. (2021) discovered that administration of arabic gum, which has a similar structure and composition to neem gum, orally for 28 days at a dose of 15% resulted in a reduction of blood glucose levels in wistar rats induced with diabetes. In addition, there are the antioxidants in neem gum, which can neutralize free radicals and thereby reduce oxidative stress caused by hyperglycemia. Moreover, Bashar et al. (2021) discovered that administering Arabic gum at a dose of 10 g orally for 30 days reduced blood glucose levels in rats with induced diabetes. This indicates that the antioxidant effect begins to work, supported by increased levels of antioxidant enzymes in the form of superoxide dismutase (SOD) and catalase (CAT).
Thus far, no research has examined the therapeutic effects of neem gum on blood glucose levels in diabetic conditions. Therefore, the researchers felt the need to further study the effects of neem gum on blood glucose levels in diabetic rat models. The aim of this study was to determine the effect of administering neem gum on reducing blood glucose levels in diabetic rats induced by streptozotocin.
RESEARCH METHODS
Ethical Clearance
This study used rats as experimental animals that had obtained a certificate of ethical approval from the Ethics Committee of the Faculty of Medicine, University of Jember, allowing the study to proceed. The ethical approval certificate number for this study is 1702/H25.1.11/KE/2023.
Plant Material Collection and Identification
Neem gum, often known as neem sap, is derived from the wood of the Azadiracta indica tree. Neem gum was gathered from neem farmers in Merak Hamlet, Sumberwaru Village, Banyuputih Subdistrict, Situbondo Regency, Indonesia, and was identified by the Botany Laboratory of the Faculty of Mathematics and Natural Sciences, University of Jember.
Preparation of Neem Gum Solution
The first step in the preparation of a neem gum solution is crushing the neem gum into a fine powder. 120 g of neem gum powder mixed with 160 ml of distilled water, then the mixture is homogenized until they dissolved at 40°C (Kalaskar et al., 2021). After dissolved, each dose (30, 15, 7.5, and 3.75 g/kgBW) was obtained through multilevel dilution (Firdaus et al., 2022).
Diabetes Induction
Streptozotocin (STZ) under the trademark Santa Cruz was used to induce diabetes in rats after they had fasted for eight hours. STZ was first dissolved in citrate buffer with a concentration of 0.1 M and a pH of 4.5. The dose used for STZ induction was 45 mg/kgBW
intraperitoneally (Dewi et al., 2022). After being induced, the rats were given 10% dextrose (4 cc/day) in the first 48 hours once a day using an orogastric tube (feeding tube) technique to prevent fatal hypoglycemia (Padugupati et al., 2021).
Experimental Animals and Research Design
Twenty-four white rat wistar strain (Rattus norvegicus) test animals weighing 200-300 g and aged two to three months were obtained from obtained from the Animal House, Faculty of Medicine, University of Jember. Rats were fed pellets and drank ad libitum, and they were acclimatized for seven days before being given treatment. Moreover, rats were divided into six groups using stratified random sampling techniques based on body
weight and glucose levels prior to STZ induction. The normal control group was only given distilled water, the 0 gram/kgBW dose group was given STZ at a dose of 45 mg/kgBW, the 3.75 gram/kgBW NG dose group was given STZ at a dose of 45 mg/kgBW, the 7.5
gram/kgBW NG dose group was given STZ at a dose of 45 mg/kgBW, the 15
gram/kgBW NG dose group was given STZ at a dose of 45 mg/kgBW, and the 30 gram/kgBW NG dose group was given STZ at a dose of 45 mg/kgBW. In addition, a feeding tube was used for administering neem gum orally to rats. After 28 days of treatment, the rats were terminated by injecting ketamine at a dose of 75 mg/KgBW and xylazine at a dose of 5 mg/KgBW intraperitoneally (Widyawati and Ayomi, 2020).
Measurement of Fasting Blood Glucose (FBG)
FBG measurements in rats were carried out three times. Before measuring FBG, rats fasted for eight hours. Furthermore, FBG measurements were carried out before the rats were induced by STZ, three days after STZ induction (pretest), and on the last day of treatment (posttest). If the FBG level measured after STZ induction reached ≥126 mg/dL, then proceed to the administration of neem gum solution (Wiyono et al., 2021; Dewi et al., 2022). In addition, rat blood was collected aseptically with a needle (a blood lancet) by piercing the tip of the rat’s tail. The blood collected was then attached to a blood glucose strip on an EasyTouch® glucometer to measure fasting blood glucose levels (Noena et al., 2020).
Statistical Analysis
The data obtained after treatment was tested using the SPSS application. Data analysis was carried out using paired t-test to determine the difference between the pretest and posttest of each treatment group. The interpretation of the paired t-test is that if the significance value was < 0.05,
the paired t-test indicated a significant difference.
RESULT AND DISCUSSION
Results
Table 1 shows the findings of the measurement of fasting blood glucose levels in this study. The data in Table 1 show that the average FBG before STZ induction showed normal results in all groups (≤126 mg/dL). The FBG levels of the rats in the treatment group increased more during the pretest than the posttest due to STZ induction. After STZ induction, rats with hyperglycemia (≥126 mg/dL) were administered a feeding tube of neem gum solution for 28 days. In addition, administering neem gum solutions aimed to reduce the level of FBG in diabetic rats. The group that administered neem gum and the normal control also showed lower FBG levels than the group that administered only STZ and distilled water.
The Differences in Pretest and Posttest Blood Glucose Levels
The paired t-test was used to determine whether there were significant differences in FBG levels before and after treatment (pretest and posttest). This test required that the data have a normal and homogeneous in distribution. The normality test was carried out using the Shapiro-Wilk test, while the homogeneity test used the Levene test. The results of the Shapiro-Wilk, Levene, and paired t-test tests are in Table 2.
According to this test, all groups have normal and homogeneous data (P>0.05). Following the normality and homogeneity tests in each group, a paired t-test was conducted. There was no significant difference between the pretest and posttest FBG in the normal control group and dose 0 groups (P>0.05). These results were as expected because the normal control group were not made diabetic and only given distilled water during the treatment. In dose 0 group, diabetics were created by injecting STZ 45 mg/kgBW, but no neem gum solution therapy was administered and only
distilled water through feeding tube was given; therefore, the group’s posttest FBG level was still as high as the pretest FBG. Meanwhile, FBG levels decreased significantly (P<0.05) in the 7.5, 15, and 30 gram/kgBW dose groups. One group, the 3.75 gram/kgBW dose group, did not experience a significant decrease in FBG levels (P>0.05), although the average posttest FBG decreased compared to the pretest FBG. In addition, fasting blood glucose levels in the 3.75 gram/kgBW dose group did not significantly decrease, possibly because the dose of neem gum administered during the treatment was too small, so the effect of the reduction in blood glucose levels was not as strong as in the 7.5, 15, and 30 gram/kgBW dose groups.
The Relationship Between Neem Gum Dose and Blood Glucose Levels
A regression test was used to determine the minimum and maximum effective doses of neem gum for reducing blood glucose levels. The data used was the average FBG posttest. Prior to the regression test, a normality test was conducted using the Shapiro-Wilk test. The results of the normality test showed that all groups have normal data distribution values (P>0.05).
The selected curve is a curve with a quadratic equation type. The quadratic equation type curve was chosen because it has the highest R square value, 0.970, when compared to other types of curves. From the regression test, the equation obtained is y = 1.059x2–46.576x+534. The regression curve and quadratic equation can be seen in Fig 1. According to PERKENI (2021), the normal fasting blood glucose level is 80– 126 mg/dL; it is considered diabetes if FBG ≥126 mg/dL. Thus, to determine the minimum effective dose, the value of y = 126 mg/dL was entered as the therapeutic target for treating diabetic rats. In addition, the maximum effective dose is 80 mg/dL, while the lower limit of normal fasting blood glucose levels is 80 mg/dL. According to this calculation from the equation, the minimum and maximum effective doses of neem gum for reducing
blood glucose levels are 12 and 15 mg/dL, respectively.
Discussion
According to Table 1, FBG levels increased after the rats were injected with STZ. After STZ induction, the average FBG level of the entire group was 439.7 mg/dL. This finding is in line with the research findings of Dewi et al. (2022), who discovered that rats induced by STZ at 45 mg/kg BW had an average increase in fasting blood glucose levels of 454.9 mg/dL. When FBG levels reached 126 mg/dL, rats developed diabetes. Diabetic rats were administered neem gum solution as therapy, except for dose 0 group , which was only given distilled water through the feeding tube. Furthermore, the normal control group of rats had an average pretest FBG of 98.2 mg/dL. It indicates that the rats were not diabetic because STZ was not induced in them. The mechanism by which STZ causes rats to become diabetic is due to its ability to prevent DNA synthesis in mammalian and bacterial cells, resulting in a special reaction with one of the nitrogenous bases, cytosine. The process of DNA degeneration and destruction occurs as a result of this response. Moreover, the toxic impact of STZ is more selective on pancreatic beta cells because it is based on the chemical structure of STZ, which is analogous to the glucose group, and pancreatic beta cells are more active in taking up glucose than other cells (Husna et al., 2019). Streptozotocin enters pancreatic beta cells via GLUT2 and causes DNA alkylation. Furthermore, STZ also induces the ribosylation of poly-adenosine diphosphate (ADP) and the release of nitrogen monoxide (NO) in pancreatic beta cells, resulting in an increase in ROS production (Tripathi and Verma, 2014). Continued production of ROS will exacerbate the destruction and necrosis of pancreatic beta cells and disrupt their function in secreting insulin, which plays a role in the regulation of blood glucose (Dewi et al., 2011; Tripathi and Verma, 2014).
The results of the study in Table 1 show that the administration of neem gum solution to the treatment group in all dose ranges caused a reduction in FBG levels compared to the group that was only given distilled water. The higher the dose of neem gum solution given, the lower the FBG level of the diabetic rat model will be; even in some groups, it has reached a normal FBG value of ≤126 mg/dL.
Thus far, there have been no studies that have examined the effect of the administration of neem gum on the
reduction in blood glucose levels. A study conducted by Wiyono et al. (2021),
discovered that administering Baluran
arabic gum at a dose of 15% in STZ-
induced Wistar rats at 40 mg/kgBW could reduce fasting blood glucose levels. In the 28-day study, serial fasting blood glucose was examined every 7 days, and it was discovered that blood glucose levels in the group administered gum arabic solution at a dose of 15% decreased significantly on days 14 to 28 compared to the group that was not given gum arabic solution. The administration of neem gum containing crude polysaccharides such as l-arabinose, d-galactose, rhammnose, and others has non-digestible polysaccharide properties; as a result, bacteria in the colon can ferment slowly from neem gum and produce shortchain fatty acids (SCFA). In addition, there are several products from SCFA fermentation, such as acetate (C2), propionate (C3), and butyrate (C4) (Firdaus et al., 2018). Furthermore, the fermented product of SCFA stimulates L cells in the intestinal mucosa to increase the secretion of the hormone glucagon-like peptide-1 (GLP-1). As a result, an increase in the GLP-1 hormone can activate the phosphoinositide 3-kinase (PI3K) enzyme in skeletal muscle cells, causing an increase in GLUT4 expression (Besten et al., 2013). Ultimately, increased GLUT4 expression causes glucose uptake into skeletal muscle cells to also increase (Darajat et al., 2019). Neem gum also contains other metabolites such as alkaloids, flavonoids, saponins, and
tannins, which have the potential to interfere with the work and activity of the α-glucosidase enzyme and thus help lower blood glucose levels (Metwally, 2012; Mirghani et al., 2018; Fardhani and Graciella, 2023).
The research from Babiker et al. (2017) discovered that gum arabic (Acacia Senegal), which has a similar composition to neem gum, such as l-arabinose, l-rhamnosa, flavonoids, and tannins could reduce fasting blood glucose levels and HbA1C at a dose of 30 g in 120 patients for 3 months. Neem gum, which is similar to gum arabic in composition, has the ability to reduce the expression of Na+-coupled glucose carriers in the intestinal mucosa (specifically in enterocyte cells) by reducing sodium/glucose cotransporter 1 (SGLT1). Due to decreased SGLT1 transporters, glucose transport from the intestine in diabetic rats is delayed (Nasir et al., 2010). As a result of this delay, the process of macronutrient absorption becomes slower, which is usually associated with changes in intestinal peptides that result in a reduction in postprandial blood glucose levels. A reduction in the SGLT1 transporter can also induce an increase in hunger-related hormones such as ghrelin as well as other hormones, including leptin, cholecystokinin, and glucagon-like peptide 1 (GLP-1). These hormones can reduce hunger by increasing feelings of fullness after eating through a variety of mechanisms (Steinert et al., 2017; Babiker et al., 2017).
In addition, crude neem gum polysaccharides also contain antioxidants, so they can reduce oxidative stress caused by STZ and hyperglycemia (Malviya et al., 2017; Moenim et al., 2018). The antioxidant activity of neem gum has been demonstrated through several studies that have been conducted previously using various methods of examination, including 1,1-diphenyl-2-picrylhydrazyl (DPPH), thin-layer chromatography (TLC), copper-reducing antioxidant capacity (CUPRAC),
and others (Malviya et al., 2017; Mirghani et al., 2018; Shobana et al., 2022). Moreover, the molecular structure of neem gum, which contains -COOH and -CHOH groups, can serve as a proton donor by donating hydrogen ions in the liquid phase to neutralize free radicals, thereby stopping the chain reaction (Malviya et al., 2017). Furthermore, the antioxidant activity of neem gum helps protect diabetics from oxidative stress, which can cause microvascular and macrovascular complications (Malviya et al., 2017; Moenim et al., 2018).
CONCLUSION AND SUGGESTION
Conclusion
Administering neem gum could reduce fasting blood glucose levels in Wistar rats induced by streptozotocin. It was discovered that an effective dose of neem gum to reduce blood glucose levels was between 12 and 15 grams/kg BW. In this study, there were no reported side effects during the administration of the neem gum solution. Further phytochemical studies are needed to determine the content of active metabolites in neem gum, as well as further research on the effect of neem gum on reducing blood glucose levels with serial blood glucose examinations to determine the duration of neem gum’s activity.
Suggestion
Phytochemical studies are needed to determine the active metabolites contained in neem gum and research on the effect of neem gum on reducing blood glucose levels with a dose range of 12-15 grams/kg, as well as serial blood glucose examinations to determine the working time of neem gum.
ACKNOWLEDGEMENT
Special thanks to the doctors and lecturers at the Faculty of Medicine, University of Jember, who have helped complete this research from the field stage to writing articles.
REFERENCES
Babiker R, Elmushraf K, Keogh MB, Banaga ASI, Saeed AM. 2017. Metabolic effects of gum arabic (Acacia senegal) in patients with type 2 diabetes mellitus (t2dm): randomized, placebo controlled double blind trial. Func. Foods Health Dis. 7(3): 219–231.
Bashar K, Humaidan NH, Addai ZR. 2021. Effect of gum arabic and olive leaf extract on blood sugar and antioxidant level in healthy and experimental diabetic male rats. Nat. Volat. Essen. Oils. 8(6): 532–539.
Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54(9): 2325– 2340.
Darajat A, Sakinah EN, Hairrudin. 2019. Efek kandungan serat beras analog terhadap ekspresi GLUT4 otot rangka tikus diabetes. J. Gizi Klin. Indon. 16(1): 14-21.
Dewi M, Suryono, Wulandari P. 2022. Aktivitas renoprotektif daun kelor (Moringa oleifera Lamk) pada tikus model diabetes mellitus tipe 2. Pharmascience. 9(2): 199-210.
Dewi M, Wijaya I, Wijayahadi N. 2011. Ekstrak bawang putih (Allium sativum) dan ekspresi insulin serta derajat insulitis pankreas tikus sprague dawley yang diinduksi streptozotocin. Media Med. Indon. 45(2): 105–112.
Elgebaly MM, Arreguin J, Storke N. 2019. Targets, treatments, and outcomes updates in diabetic stroke. J. Stroke Cerebrovascular Dis. 28(6): 1413–
1420.
Fardhani, IM and Graciella C. 2023. Potensi aktivitas antidiabetes daun kemangi (Ocimum basilicum):
literature review. Prepotif: J. Kes. Mas. 7(1): 564–574.
Firdaus J, Febianti Z, Hidayat MRF, Sakinah EN. 2022. Efek neem gum (Azadirachta indica) terhadap kadar
sgot sgpt tikus wistar yang diinduksi diazinon. J. Nut. College. 11(3): 258263.
Firdaus J, Sulistiyaningsih E, Subagio A. 2018. Resistant starch modified cassava flour (MOCAF) improves insulin resistance. Asian J. Clin. Nutr. 10(1): 32-36.
Hameed S, Kumar P, Kumar M, Mohan L, Dikshit, H. 2022. Evaluation of suspected adverse drug reactions of oral anti-diabetic drugs in a tertiary care hospital of Bihar, India: an
observational study. Panacea J. Med. Sci. 12(1): 172–176.
Husna F, Suyatna, FD, Arozal W, Purwaningsih EH. 2019. Model hewan coba pada penelitian diabetes. Pharm. Sci. Res. 6(3): 131–41.
Indonesian Society of Endocrinology
(PERKENI). 2021. Management and prevention of type 2 diabetes mellitus in adults in Indonesia. PB PERKENI.
International Diabetes Federation. 2021. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes
Federation.
Kalaskar MG, Mutha RE, Tatiya AU, Firke SD, Surana SJ, Dhoka KA, Heda K. 2021. Purification and modification of neem gum for enhancement of its suspending property. Futur. J. Pharm. Sci. 7: 114.
Malviya R, Sharma PK, Dubey SK. 2017. Antioxidant potential and emulsifying properties of neem (Azadirachita indica, family meliaceae) gum
polysaccharide. Pharm. Anal. Acta. 8(8): 1-7.
Metwally N. 2012. Chemical constituents of the egyptian plant anabasis articulata (forssk) moq and its antidiabetic effects on rats with streptozotocin-induced diabetic hepatopathy. J. Appl. Pharm. Sci. 2(4): 54–65.
Ministry of Health of the Republic of Indonesia. 2020. Diabetes Situation and Analysis. Center for Data and Information of the Ministry of Health of the Republic of Indonesia.
Mirghani MES, Elnour AAM, Kabbashi NA, Alam Z. 2018. Determination of antioxidant activity of gum arabic: an exudation from two different locations. Sci. Asia. 44(3): 179–186.
Moenim ME, Hassan EA, Osman ME. 2018. Characterization and rheological behavior of neem gum (Azadirachta indica). Int. J. Chem. Stud. 6(3): 1977– 1981.
Nasir O, Artunc F, Wang K, Rexhepaj R, Föller M, Ebrahim A, Kempe DS, Biswas R, Bhandaru M, Walter M, Mohebbi N, Wagner CA, Saeed AM, Lang F. 2010. Downregulation of mouse intestinal Na (+) coupled glucose transporter SGLT1 by gum arabic (Acacia Senegal). Cellular
Physiology and Biochemistry: Int. J. Exp. Cell. Physiol. Biochem.
Pharmacol. 25(2-3): 203–210.
Noena RAN, Thahir Z, Base NH, Fahriani. 2020. Aktivitas antihiperglikemia
minyak kluwak pada hewan uji mencit (Mus musculus). J. Kes. Yamasi
Makassar. 4(1): 40–46.
Padugupati S, Rhamoorthy S, Thangavelu K, Sharma D, and Jamadar D. 2021. Effective dose of streptozotocin to induce diabetes mellitus and variation of biophysical and biochemical parameters in albino wistar rats. J. Clin. Diag. Res. 15(10): 1–5.
Putra RJS, Achmad A, Rachma HP. 2017. Kejadian efek samping potensial terapi obat anti diabetes pasien diabetes melitus berdasarkan algoritma naranjo, Pharm. J. Indon. 2(2): 45–50.
Shobana N, Prakash P, Samrot AV, Cypriyana PJ, Kajal P, Sathiyasree M, Saigeetha S, Dhas TS, Anand DA, Sabesan, GS, Muthuvenkatachala BS,
Mohanty BK, Visvanathan S. 2022. Purification and characterization of gum-derived polysaccharides of moringa oleifera and Azadirachta indica and their applications as plant stimulants and bio-pesticidal agents. Molecules. 27(12): 3720.
Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N.
2017. Ghrelin, CCK, GLP-1, PYY (336): secretory controls and
physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 97(1): 411-463.
Tripathi V, Verma J. 2014. Different models used to induce diabetes: a comprehensive review article. Int. J. Pharm. Pharm. Sci. 6(6): 29-32.
Widyawati R, Ayomi BDS. 2015. The comparison of ketamine, xylazine, and ketamine-xylazine xombination to rat (Rattus norvegicus). J. Vitek Bidang Ked. Hewan. (5): 42–45.
Wiyono HT, Utami ET, and Wardhani DWP. 2021. Effect of baluran gum arabic on blood glucose level in diabetic rat (Rattus novergicus). Berkala Sainstek, 9(2): 81-85.
World Health Organization. 2018. Noncommunicable Diseases Country Profiles. https://www.who.int/news-room/fact-sheets/detail/noncommunicable diseases. [Diakses pada 12 November 2022].
Wulandari P, Ramadani AF, Suryono, Santosa A. 2022. Effective dose of moringa leaf extract (Moringa oleifera Lamk.) to descrease total cholesterol levels in streptozotocin-induced male wistar rats. J. Agromed. Med. Sci. 8(2): 102-107.
Table 1. The Average Fasting Blood Glucose Levels
|
Dosage group (gram/kgBB) |
GDP pre STZ induction (mg/dL) |
GDP pretest (mg/dL) |
GDP posttest (mg/dL) |
|
Normal control |
88,5±22,4 |
98,2±14,4 |
78,2±5,3 |
|
Dose 0 |
91,5±5,3 |
471,2±124,1 |
542,6±38,8 |
|
Dosage 3.75 |
99,2±8,6 |
496,7±74,1 |
358,6±22,3 |
|
Dosage 7.5 |
111,2±11,6 |
532,0±48,6 |
188,5±29,2 |
|
Dosage 15 |
102,5±19,1 |
535,0±89,1 |
105,0±12,3 |
|
Dosage 30 |
107,0±19,1 |
505,5±125,1 |
85,3±1,5 |
|
Mean |
99,9±8,7 |
439,7±168,9 |
226,4±187,3 |
Table 2. The Results of the Shapiro-Wilk, Levene, and Paired t-test Tests on FBG Levels
Dosage group Shapiro Wilk Levene's Paired T-Test
(gram/kgBB)
|
Normal control |
0,200 |
0,299 |
0,720 |
|
Dose 0 |
0,231 |
0,146 |
0,140 |
|
Dosage 3.75 |
0,153 |
0,097 |
0,237 |
|
Dosage 7.5 |
0,530 |
0,377 |
0,001 |
|
Dosage 15 |
0,150 |
0,051 |
0,002 |
|
Dosage 30 |
0,130 |
0,087 |
0,002 |
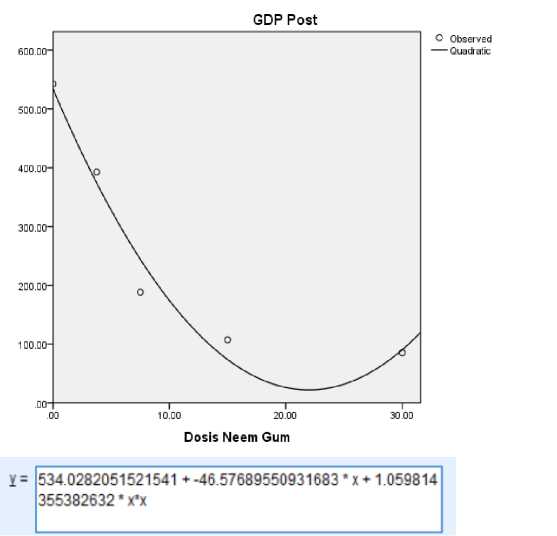
Figure 1. Quadratic Regression Test Curve Between Neem Gum doses and Posttest FBG levels
990
Discussion and feedback