DTA-TG Analysis of Gd0.95La0.05Ba1.95Sr0.05Cu3Oy Compounds
on
Buletin Fisika Vol 21 No. 1 February 2020 : 33 – 36
DTA-TG Analysis of Gd0.95La0.05Ba1.95Sr0.05Cu3Oy
Compounds
M. Sumadiyasa1*, I P. Suardana2, N. Wendri3
-
1,2,3Jurusan Fisika, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Udayana, Kampus Bukit Jimbaran, Badung, Bali, Indonesia 80361
Email: sumadiyasa@unud.ac.id*, suardanaputu@unud.ac.id, wendri@unud.ac.id
Abstrak – The sintering temperature is played a vital role in the evolution of phase structure, microstructure, and the properties of the superconductor. In this study, the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase compound has been synthesized by the wet method using HNO3 as a solvent. The samples were divided into two groups. The first sample was calcined at 400 °C for 2 hours + 500 °C for 2 hours + 600 °C for 6 hours. The second sample treated by the same process and then continued by heating at 900 °C for 15 minutes. The effect of the calcination temperature for the synthesis of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ bulks was investigated using the DTA-TG method. The results showed that the optimum reaction temperature for the formation of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase was 938 °C. The additional heating temperature e.g. 900 °C for 15 minutes on the calcination process can reduce the optimum formation temperature of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ compounds by 20 °C. The peritectic melting reaction
temperatures of the sample without the addition of heating and with the addition of heating at temperature 900 °C for 15 minutes are 1032°C and 1035°C, respectively. The melting temperatures of both samples are 1164 °C and 1200 °C.
Keywords: DTA-TG method, calcination temperature, sintering temperature, Gd0.9La0.1Ba1.95Sr0.05Cu3 O7-δ compounds, wet method
was given heating at 900 °C for 15 minutes. This final powder as a precursor is subjected to high-temperature heat treatments (sintering) for production of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ compound.
To studies the thermal properties, the sample has been characterized by using of DTA-TG of STA 1600C. The precursor sample was heated at a maximum temperature of 1203 °C with an air atmosphere with flow velocity 0.5/minute and at a rate of 600 °C/hours.
Figure 1 is the result DTA-TGA measurement for Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ precursor without heating at 900 °C. From Figure 1, the TGA curve at temperature between 168-1200 °C shows a decrease in the total mass of 26%. At temperatures between 168-668 °C show a mass decrease of 13%. From the DTA curve there was a complex reaction in which there was evaporation of nitrates and decomposition of metal-nitrate salts. At temperatures between 668-954 °C a mass decrease of 10% occurred. From the DTA curve, the peak at 938 °C is observed, according some researchers have previously shown that the Gd1Ba2Cu3O7-δ phase can form at temperatures between 850-950 °C [4-6], therefore we conclude that Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase can be formed at temperature 938 °C. On the TG curve, a 3% mass decrease is observed at temperatures between 954-1200 °C, whereas on the DTA curve the peak at 1032 °C was observed.
The GBCO-123 family superconductor has a peritectic melting temperature at temperatures between 980-1090 °C [7, 8, 9]. Prado et al also reported that the endothermic DTA peaks under pure oxygen corresponding to the peritectic melting reaction which takes place at 1073 °C [10] and the actual peritectic temperature of GdBCO bulk superconductors, i.e. 1030 °C [11]. Therefore, we suspect a temperature at 1032 °C is a peritectic melting reaction temperature of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase. Furthermore, the DTA curve drops sharply at temperature 1164 °C, we suspect that at this temperature a complete melting of the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase has occurred.
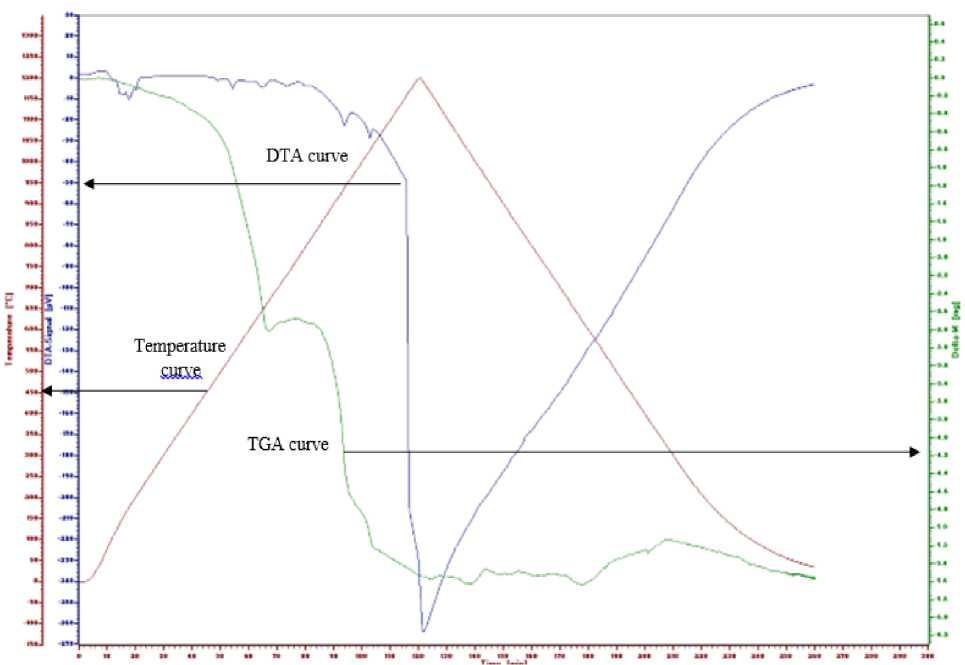
Figure 1. DTA-TG measurement result of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ precursor without heating at 900 °C. Initial of sample is 21.3 mg.
The result DTA-TGA measurement of the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ precursor with heating at 900 °C for 0.25 hours as of Figure 2. From Figure 2, the TGA curve between 168-1200 °C shows a decrease
in the total mass of 23%. At temperature between 2 °C to 777 °C there was a mass reduction of 12%, the DTA curve showed a widen peak with a peak at 285 °C and a small peak at 750 °C, it is we suspect a complex reaction in which nitrate evaporation and decomposition of metal-nitrate salts occurred. At temperatures between 777 °C to 962 °C there was a mass decrease of 9%. From the DTA curve, the peak at 918 °C was observed. According some researchers have previously shown that the Gd1Ba2Cu3O7-δ phase can form at temperatures between 900-950 °C [4-6], therefore we suspect that the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase can be formed at optimum temperature 918 °C. On the TG curve, 2% mass decrease is observed too at temperatures between 962-1200 °C, whereas on the DTA curve the peak at 1035 °C was observed. We suspect it is the peritectic melting reaction temperature of the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ. Furthermore, the DTA curve drops sharply, we suspect that the melt complete of the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase occurring at temperatures above 1200 °C.
From the above explanation it can be concluded that there is a difference reaction temperature for formation of the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase. For samples calcined without heating and by heating at 900 °C i.e. at 938 °C and 918 °C respectively.
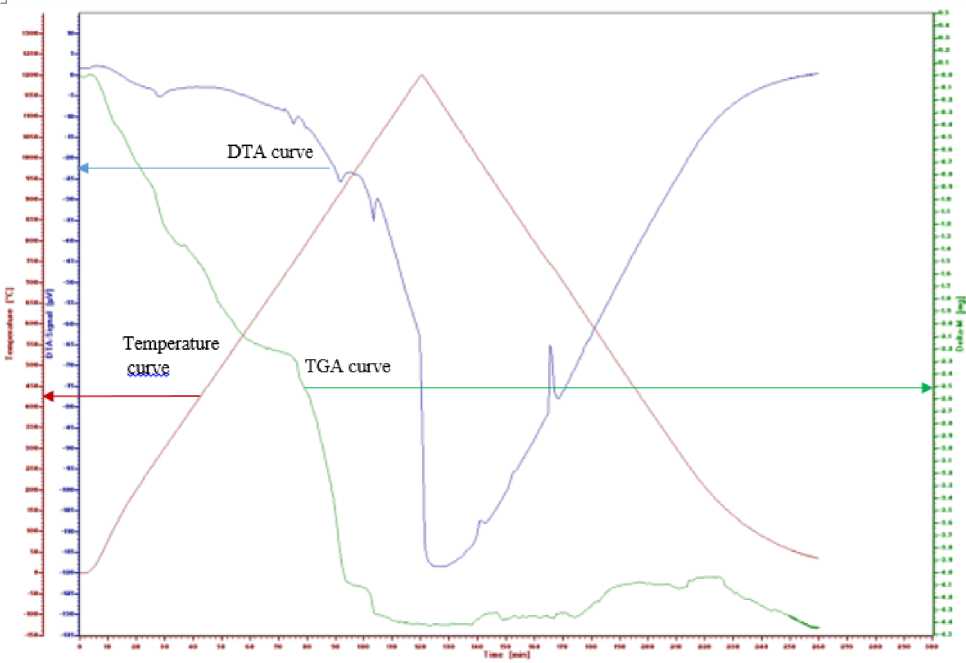
Figure 2. DTA-TG measurement result of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ precursor with heating at 900 °C for 0.25 hours. Initial of sample is 20.7 mg.
Calcination affects the reaction temperature of the formation of Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ compounds. The reaction temperature optimum for the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase formation is 938 °C and peritectic melting reaction temperature at 1032 °C for calcined samples without heating at 900 °C. The reaction temperature optimum for the Gd0.9La0.1Ba1.95Sr0.05Cu3O7-δ phase formation is 918 °C and peritectic melting reaction temperature at 1035 °C for calcined samples accompanied by heating at 900 °C.
Acknowledgements
This work is a part of the fundamental research 2019 grand. The authors are thankful to RISTEK DIKTI and LPPM of Udayana University.
References
-
[1] Fadila Taïr, Laura Carreras, Jaume Camps, Jordi Farjas, Pere Roura, Albert Calleja, Teresa Puig, Xavier Obradors, Melting temperature of YBa2Cu3O7-δ and GdBa2Cu3O7-δ at subatmospheric partial pressure, Journal of Alloys and Compounds, 692, 2017, pp. 787–792.
-
[2] J H Durrell, A R Dennis, J Jaroszynski, M D Ainslie, K G B Palmer, Y-H Shi, A M Campbell, J Hull, M Strasik, E E Hellstrom and D A Cardwell, A trapped field of 17.6T in melt-processed, bulk Gd-Ba-Cu-O reinforced with shrink-fit steel, Supercond. Sci. Technol. 27, 2014, pp. 082001
-
[3] Made Sumadiyasa, I. Gusti Agung Putra Adnyana, Nyoman Wendri, Putu Suardana, Synthesis and Characterization of GLBCO-123 Phase: Gd1-xLxBa2Cu3O7-d (x = 0.0 - 0.5), Journal of Materials Science and Chemical Engineering, 5, 2017, pp. 49–57
-
[4] W. Wong-Ng, L. P. Cook, H. B. Su, M. D. Vaudin, C. K. Chiang, D. R. Welch, E. R. Fuller, Jr, Z. Yang, and L. H. Bennett, Phase Transformations in the High-T Superconducting Compounds, Ba2RCu3O7–δ (R= Nd, Sm, Gd, Y, Ho, and Er), J. Res. Natl. Inst. Stand. Technol, 111, 2006, pp. 41–55
-
[5] Yustinus Purwamargapratala, Didin Swinatapura, Engkir Eukirman, Influence of Temperature and time Sintering to forming of superconductor, Indonesian Journal of Materials Science, special issue, 2007, pp. 77–82
-
[6] K. Matsushima, C. Taka and A. Nishida, Variations of superconducting transition temperature in YbBa2Cu3O7-d ceramics by Gd substitution, IOP Conf. Series: Journal of Physics: Conf. Series, 969, 2018, pp. 012059.
-
[7] Devendra K Namburi, Yunhua Shi, Anthony R Dennis, John H Durrell and David A Cardwell, A robust seeding technique for the growth of single grain (RE)BCO and (RE)BCO–Ag bulk superconductors, Supercond. Sci. Technol. 31, 2018, pp. 1–10
-
[8] Y. Shi, N. Hari Babu, K. Iida, and D. A. Cardwell, Growth Rate and Superconducting Properties of Gd-Ba-Cu-O Bulk Superconductors Melt Processed in Air, IEEE Transactions on Applied Superconductivity, 17, 2007, pp. 2984–2987
-
[9] Jens Christiansen, Ceramic High Temperature Superconductors for High Current Applications, Dissertation, Institute of Mineral Industry, Technical University of Denmark, 1996
-
[10] F. Prado, A. Caneiro, A. Serquis, High temperature thermodynamic properties, orthorhombic-tetragonal transition and phase stability of GdBa2Cu3Oy and related R123 compounds, Physica C, 295, 1998, pp. 235–246
-
[11] Y. Nakanishi, S. Pavan Kumar Naik, M. Muralidhar and M. Murakami, Effect of growth temperature on properties of bulk GdBa2Cu3Oy superconductors grown by IG process, IOP Conf. Series: Journal of Physics: Conf. Series, 871, 2017, pp. 012052.
36
Discussion and feedback