THE MORPHOMETRY AND SEX RATIO OF Gerris marginatus Say (HETEROPTERA: GERRIDAE) IN TROPICAL AREA
on
Jurnal Bumi Lestari, Volume 15 No. 2, Agustus 2015, hlm. 184-188
THE MORPHOMETRY AND SEX RATIO OF Gerris marginatus Say (HETEROPTERA: GERRIDAE) IN TROPICAL AREA
Ni Luh Watiniasih
Department of Biology, Faculty of Mathematic and Basic Science Udayana University, Jimbaran, Bali 80361 Email: luhwatiniasih@unud.ac.id
Abstract
Gerris marginatus is one fresh water insect distributed from temperate to tropical areas. It can be found in almost every freshwater biotope, making them excellent objects for ecological and biogeographic studies. Most recent studies found that this water insect was one good candidate for examining the effect of fresh water pollution. This species shows morphometric differences between male and female, but there has no studies examining this morphometric differences particularly in tropical areas. This study aimed to determine and investigate the morphometric differences between male and female and their sex ratio of G. marginatus in one of freshwater body in Bali. Samples were collected from Jangu River at Karangasem Regency on February 2014. Samples were preserved in 70 % awaiting for identification and further studies. Identification was performed at Animal Taxonomy Laboratory, Biology Department. The results show that there were morphometric differences between male and female of G. marginatus in tropical area. Males were significantly larger of many variable measured compared to females. However, they also exhibit sex ratio bias toward females.
Keywords: morphometric, sex ratio, water strider, Gerris marginatus
Insect is the most divers organism found in the world. They can be found in all types of habitats, such as terrestrial and water habitats. However, the distribution capability was, in some point, affected by their microhabitats and ability to distribute such as in Hemipteran ( Carbonell et al., 2011) . Water striders or also known as water skipper are insects commonly found on most bodies of fresh water, such as in small streams to largerivers, ponds, and lakes, and even on the surface of the ocean. Their long legs distribute the body weight, enabling them to “walk” or “skate” on the surface film (Stonedahl and Lattin, 1982). Body size variation of this insect was observed in natural environment, however it was no information to what extent the difference of the body size would affect its gender, which was a concern of this study.
Phenotypic differences between male and female individual of Gerris may have a specific function that
may drive by coevolutionary process. It has been found that wing polymorphism is widespread in the Gerridae. Among population, the univoltine populationscan betotally apterous or totally macropterous. Apterous populations are generally restricted to stable aquatic habitats (rivers, large lakes), while macropterous populations inhabit less dependable water supplies (seasonal streams, ponds, small lakes). Brinkhurst (1963) suggests that apterous popula-tions produce occasional winged forms for dispersal purposes. Bivoltine Gerris populations are frequently dimorphic during the summergeneration found in temperate environment. The ability of this insect to inhabitin a wide range of environment was suggested as an important bioindicator insect of water polutionin tropical area (Juliantara et al., 2015). This research aim to investigate the morphometric differences between male and female Gerris marginatus that has not ever been studied particularly in tropical aquatic habitat.
Samples of Gerris marginatus were collected from 2 population at Janga River, District of Karangasem Bali. Sampel were preserved in 70% alcohol and transported to Animal Taxonomy Laboratory, Department of Biology, Faculty of Mathematics and Science Faculty, Udayana University at Jimbaran Campus for the analysis. Sample sexing was conducted by observing its genital partsat the end of the individual abdomen which easily recognized by naked eye (Figure 2). Ten samples from each sex were identified. Morphological measurement of leg lengths was conducted using millimeter ruler which was measured from the most end of femur to the tip of claw. Other morphological characters such as head width, the length of rostrum, antennae, pro-thorax, thorax, and abdomen were measured under the ‘Lyca’ dissecting microscope by employing the digital “OptiLabTM Viewer 2.2” by Miconos. Measurement was performed from the picture taken employing tools provided by OptiLabTM package.Head width was measured between outer parts of both eyes. The length of rostrum was measured from the length of labrum, the length of antenna was measured from the basal to the tip of antenna, the length of pro-thorax, thorax and abdomen were measured from the most front part to the end of each character. Samples were analysis in IBM SPSS Statistics 20.
The average number of females Gerris marginatuscollected from two population was higher than the number of male (Figure 1), which was slightly bias to female (1 male : 1.1 females).
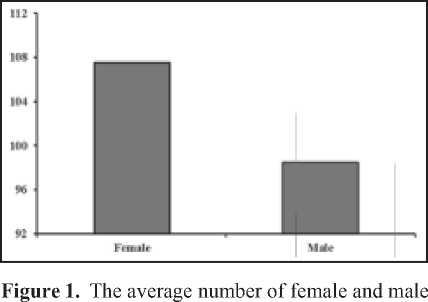
G. marginatus collected from two populations.
The result shows that morphologically, male and female genital of Gerris marginatus were very distinct (Figure 2), which can be clearly observed with naked eye. As described by Chang and Jablonski (2009), pre-genital segment of female is concave and the genitalia exposed, which will be easily available for mating. Differences in morphological features between male and female genitalia in water strider is classic that male will force female to mate. Chang and Jablonski (2009), however, found that female genitalia was partly concealed, which was contributed to the existence of coercive mating system in Gerridae.Some female Gerridae develop abdominal spines in the last abdominal segment (Ronkainen et al., 2005) and bend their abdomen downward. It was also found that to avoid forced mating,female defense themselves by jumping or rubbing the males (Arnqvist and Rowe, 2002), and the female was found to have internal reproductive adaptation (Chang and Jablonski , 2009). Evolutionary, the differences in genitalia characteristics between female and male water strider would evolved following one another. The male following this evolution also bending its abdomen and genital morphology downward (Ronkainen et al., 2005), therefore increase coercive mating initiation (Harari et al., 1999).Specific morphological trait of male genitalia on Gerris may also contribute to the reproductive success of this species. Long period of copulation time (1.5h) was argued to increase reproductive success of a male to the others (Rubenstein, 1989). Sexual dimorphic traits of a species is thought a driving force of evolution.
Morphometric measurement of characters found on Gerris marginatus was all significant different between female and male. Male was significantly larger than female in almost all character measured. Summary of the differences between female and male was presented in Table 1. The length of rostrum was longer (2.00±2.03 mm) in male compared to female (1.65±0.45 mm), as the length of antennae was longer on male (12.69±0.25 mm) than female (10.72±0.03 mm) (Figure 3). Dimorphic antennae with elaboration of traits found in Rheumatobates rileyi function to grasp female on pre-mating period, and the reduction grade of grasping traits was observed reducing mating success on male water strider (Khila et al., 2012). (Juliantara IKP, Watiniasih NL, Kasa IW, 2015)
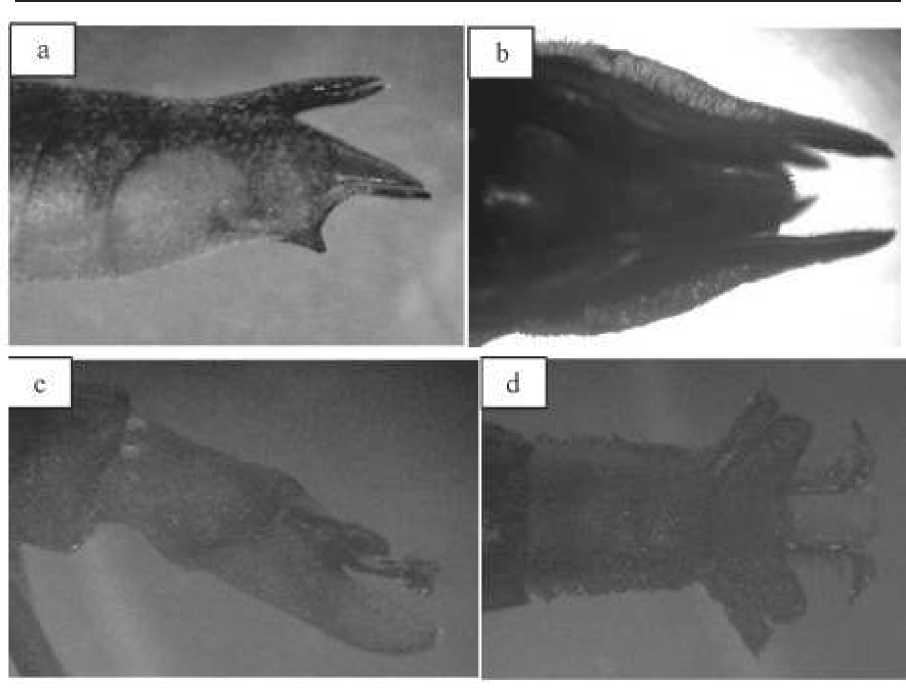
Figure 2. Morphology of lateral (a) and dorsal (b) views of female genitalia and lateral (c) dan dorsal (d)views of male genitalia (All pictures were 10x the natural size).
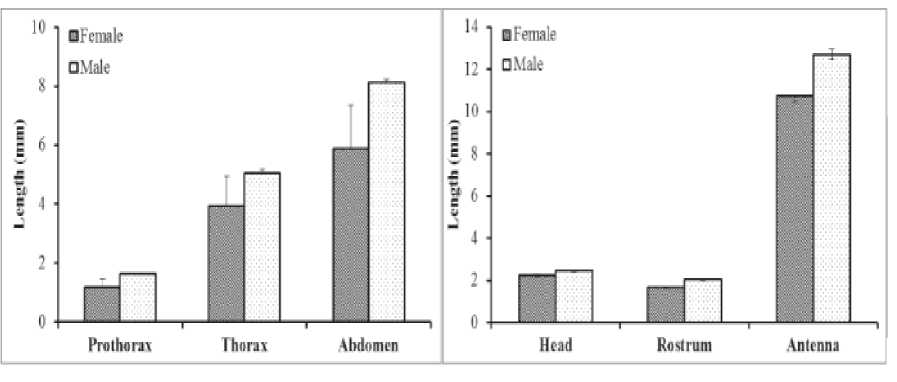
Figure 3. The length differences female and male of pro-thorax, thorax, abdomen, headwdth, rostrum and antennae of water strider (Gerris marginatus).
Table 1. The result of one way analysis of variance comparing the water strider characters of female and male.
|
Between Group ( in mm) |
Sum of Squares |
df |
Mean Square |
F |
Sig. |
|
Head width |
0.125 |
1 |
0.125 |
8.272 |
0.021 |
|
Length of Rostrums |
0.314 |
1 |
0.314 |
64.834 |
0.001 |
|
Length of Antennae |
9.643 |
1 |
9.643 |
61.667 |
0.001 |
|
Length of Forelegs |
46.513 |
1 |
46.513 |
109.8 |
0.001 |
|
Length of Middlelegs |
238.05 |
1 |
238.05 |
58.179 |
0.001 |
|
Length of Hindlegs |
316.012 |
1 |
316.012 |
68.844 |
0.001 |
|
Length of Prothorax |
0.05 |
1 |
0.05 |
5.795 |
0.047 |
|
Length of Thorax |
0.035 |
1 |
0.035 |
0.141 |
0.719 |
|
Length of Abdomen |
1.349 |
1 |
1.349 |
6.745 |
0.036 |
Female pro-thorax, thorax and abdomen were also longer on male than female. The length of prothorax was slightly but significantly shorter on female (1.17± 0.29 mm) than male (1.62± 0.02 mm) and the length of female abdomen was also shorter (5.87±1.47 mm) than male (8.11±0.11 mm). All body of Gerris was covered by hairs (microtrichia) to provide colorful glossy looked and resistant to wetting. Similarly, the length of appendages was longer on male water striders than female. The forelegs, middle legs and hind legs were all almost one fifth longer on male than on female (Figure 4).
The long legs (Arnqvist, 1992)which have been found distribute the body weight, enabling them to “walk” or “skate” on the surface film (Stonedahl and Lattin, 1982). Mating berhavior of Gerris marginatus was discuss in many papers (Arnqvist G, 1997; Stonedahl and Lattin, 1982; Arnqvist G and Rowe L,
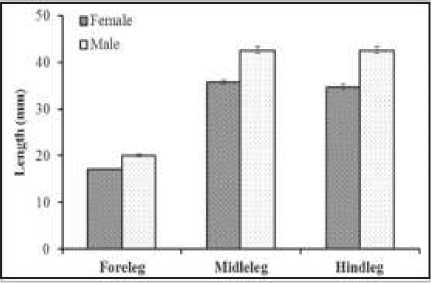
Figure 4. The length differences of female and male appendages of water strider (Gerris marginatus).
1995; Han and Jablonski PG, 2009). The mating benavior of this insect was cruel in that male will force female to mate by inserting his genetalia in order to transfer sperm (Han and Jablonski, 2009). Differences in morphometric of morphological characters in Gerris may also contribute to the coevolutionary process (Carbonell JA, Gutierrez-Canovas C, Bruno D, Abellan P, Velasco J, and Millan A., 2011)
Acknowledgement
Thanks to I Ketut Juliantara S.Pd., M.Si. for his contribution on sample collection.
Daftar Pustaka
Carbonell JA, Gutierrez-Canovas C, Bruno D, Abellan P, Velasco J, and Millan A. (2011). Ecological factors determining the distribution and assemblages of the aquatic Hemiptera (Gerromorpha & Nepomorpha) in the Segura River basin (Spain). Limnetica, 30 (1), 59-70.
Arnqvist G. (1992). Pre-copulatory fighting in water strider: inter-sexual conflict or mate assessment. Anim. Behav. 43, 559-567.
Arnqvist G. (1997). The evolution of water strider mating system:causes and consequaences of sexual conflicts. In J. C. Crispy, The Evolution of Mating System in Insects and Arachnids (pp. 146-163). Cambridge: Cambridge University Press.
Arnqvist G and Rowe L. (1995). Sexual Conflict and Arms Races between the Sexes: A
Morphological Adaptation for Control. Proceedings: Biological Sciences, 261, (1360) (Jul. 22, 1995), pp. 123-12 (pp. 123-127). The Royal Society.
Arnqvist G, Rowe L. (2002). Correlated evolution of male and female morphologies in water striders. Evolution 56, 936–947.
Busha JWM, Hub DL and Prakash M. (2008). The integument of water-walking Arthropods: form and function. Advances in Insect Physioogy 34: , 117-192.
Drake CJ, Harris HM. (1928). Concerning some North American waterstriders with descriptions of three new species. Ohio Journal of Science 28 (5), 268-276.
Han CS, Jablonski PG. (2009). Female Genitalia Concealment Promotes Intimate Male. PLoS ONE 4(6): e5793. doi:10.1371/, 1-10.
Harari A, Handler A, Landolt P . (1999). Size-assortative mating, male choice and female
choice in the curculionid beetle Diaprepes abbreviatus. Animal behaviour:58, 1191–1200.
Juliantara IKP, Watiniasih NL, Kasa IW. (2015). The toxicity of detergent and artificial color to water strider (Gerris marginatus). Jurnal Biologi FMIPA Universitas Udayana, in press.
Khila A, Abouheif E, Rowe L. (2012). Function, Developmental Genetics,and Fitness Consequences of a Sexually Antagonistic Trait. Science 336:, 585-587.
Ronkainen K, Kaitala A, Huttunen R. (2005). The effect of abdominal spines on female mating frequency and fecundity in water strider. J Isect Behav 18:, 619-631.
Rubenstein DI. (1989). Sperm competition in the water strider, Gerris remigis. Anim. Behav:38, 631-636.
Stonedahl GM, Lattin JD. (1982). The Gerridae or water strider of Oregon and Washington (Hemiptera:Heteroptera). Corvallis Oregon: Agricultural Experiment Station Oregon State University.
188
Discussion and feedback