Checklist of gastropoda from Lombok Island and its satellite islands
on
JURNAL BIOLOGI UDAYANA 24(2): 72-86
P ISSN: 1410-5292 E ISSN: 2599-2856
Daftar jenis gastropoda dari Pulau Lombok dan pulau-pulau satelitnya
Checklist of gastropoda from Lombok Island and its satellite islands
Nova Mujiono*
Research Center for Biology, Indonesian Institute of Sciences (LIPI).
Cibinong Scince Center, Jl. Raya Jakarta-Bogor Km 46, Cibinong, 16911.
*Email: nova.mzb@gmail.com
Diterima 5 Juni 2020 Disetujui 4 September 2020
ABSTRACT
Lombok, an island that lies in the Lesser Sunda Islands, is popular for the tourist destination. One attempt to promote tourism is throcugh the biodiversity of its fauna. This study aims are to summarize the diversity of gastropoda and made a checklist to make it easier studied. Museum collections and twenty-four works of literature were used as the data source. A total of 73 families and 292 species of gastropoda were documented from 29 locations. About 29% of them have their representation in the museum collections. The list dominated by marine (65%), following by terrestrial (15%), mangrove (6%), and freshwater species (5%). Twenty-two species are new records for Lombok Island. The most diverse family, Neritidae, which occupies wider habitat is represented by nine percent. Littoraria scabra is the most widely distributed species which occurs in 12 locations. The five most diverse locations were marine habitats which popular for tourism destinations. This study contributes to the development of marine ecotourism in Lombok.
Keywords: diversity, gastropoda, Lombok, marine ecotourism
INTISARI
Lombok, pulau yang terletak di Kepulauan Sunda Kecil, terkenal sebagai tujuan pariwisata. Salah satu usaha untuk mempromosikan pariwisata ialah melalui keragaman faunanya. Penelitian ini bertujuan untuk merangkum keragaman gastropoda dan membuat satu daftar jenis agar mudah untuk dipelajari. Koleksi museum dan 20 pustaka digunakan sebagai sumber datanya. Total, 73 suku dan 292 jenis gastropoda didokumentasikan dari 29 lokasi. Sekitar 29% darinya terwakili di koleksi museum. Daftar ini didominasi oleh jenis laut (65%), diikuti darat (15%), bakau (6%), dan air tawar (5%). Duapuluh dua jenis merupakan rekaman baru untuk Pulau Lombok. Neritidae dengan mencakup habitat yang lebih luas menjadi suku yang paling beragam dengan mewakili 9% seluruh jenis. Littoraria scabra ialah jenis yang paling tersebar luas dengan hadir di 12 lokasi. Lima lokasi dengan keragaman tertinggi merupakan habitat laut yang telah terkenal menjadi tujuan pariwisata. Penelitian ini turut berkontribusi terhadap pembangunan pariwisata bahari di Lombok.
Kata kunci: keragaman, gastropoda, Lombok, wisata bahari
INTRODUCTION
Lombok, an island just located to the east of the most popular island in Indonesia, Bali island. They separated by a narrow deep strait namely Lombok Strait. With the total wide area of 4.619 km2, Lombok is the second biggest island after Sumbawa (15.255 km2) and both are in the same province, Western Nusa Tenggara (Monk et al., 2000). Similar to Bali, the neighboring island, Lombok also popular as the tourist destination. Most likely for their famous beaches, such as Senggigi, Kuta, Gili Islands, and Labuhan. Another famous destination is Mountain Rinjani National Park. Gili Islands is popular for its coral’s life, Over 3.500 species of marine species life around it, compared with 1,500 off the Great Barrier Reef (Ascui & Seow, 2005).
One way to promote tourism is through biodiversity studies (Bashar, 2018). The diversity of flora and fauna in Lombok Island is already documented. The wild mammals of Lombok island are already published in 1990. It consists of 53 mammal species (Kitchener et al., 1990). Checklist flora of Lombok also already published in 2020. It consists of 1.309 species, subspecies, and varieties. The checklist in this book is divided into nine parts based on the phylogenetic classification (Rustiami et al., 2020). Meanwhile, the diversity of gastropoda in Lombok Island is never been summarized.
Gastropoda, a class of Mollusca, has been through a long period of evolution. Their ancestor was from marine form who emerged from the bottom of the sea and then invading river, lake, and land. It is also the most diverse class with a total of 32.569 known species. The most diverse gastropods are marine taxa (17.481), following by terrestrial taxa (11.116) then by freshwater taxa (3.972) (Strong et al., 2008; Rosenberg, 2014). The first person who studied mollusca of Lombok was Edgar A Smith, a British malacologist who published his list of the land snail of Lombok including the descriptions of twelve new species. The recent study by Candri et al., (2020) was documenting molluscan communities in the southern part of Lombok Island. The aims of this
paper are to summarized the data on the diversity of gastropods from Lombok Island and made a checklist for professional taxonomists or any other enthusiasts who studying gastropods. Their potency also will be discussed briefly. Hopefully, biodiversity data can promote the ecotourism activities of Lombok Island.
MATERIALS AND METHODS
This study was carried out between January and May 2020. Two types of data were used: 1) Primary data: list of species in the catalog books from the Malacology Laboratory, Museum Zoologi Bogor (MZB-LIPI), 2) Secondary data: list of the species from the works of literature (online and printed).
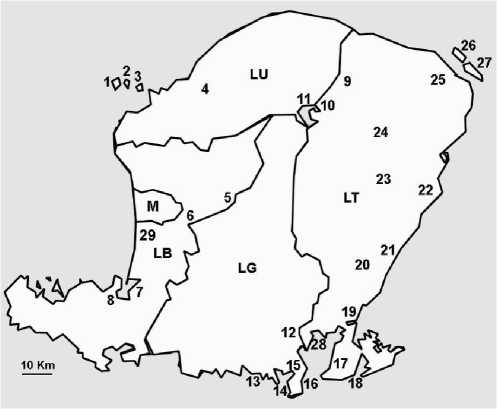
Figure 1. Administrative map of Lombok Island, with the locations of species listed in this paper. M Mataram, LB Lombok Barat, LU Lombok Utara, LT Lombok Timur, LG Lombok Tengah, 1 Gili TrawanganMR, 2 Gili MenoMR, 3 Gili AirMR, 4 GanggaFW MG, 5 SuranadiFW, 6
NarmadaFW TR, 7 LembarMG, 8 SekotongMG, 9 SembalunTR, 10 PlawanganTR, 11 Segara
AnakFW TR, 12 Praya TimurMG, 13 Pantai KutaMR, 14 Tanjung AanMR, 15 PujutFW MG TR, 16 Pantai GerupukMR, 17 JerowaruMG, 18 Teluk EkasMG MR TR, 19 KeruakFW MG , 20 LepakTR, 21 Labuan HajiFW, 22 PringgabayaFW, 23 AikmelTR, 24 SuwelaTR, 25 SambeliaFW MG, 26 Gili
LawangMG MR TR, 27 Gili SulatMG MR, 28
Batunampar MG MR, 29 Taman AyuFW. Note: FW(freshwater), MG(mangrove), MR(marine),
TR(terrestrial).
Twenty-four works of literature were examined. Together with the specimens from MZB, 29 localities were documented (Figure 1). The specimens’ collectors were sometimes also the authors, except HB Munaf and A Saim, A Hanna, W Kastoro & Maryoto, DG Reid, Y Apriyanti. Collector names, years of the publications, the locations visited and the number of species found is listed below (locations are not translated into English):
-
1. EA Smith. 1898. Lombok. 25 spesies (based on the A Everett’s collection).
-
2. B Rensch. 1931 (Segara Anak, Sembalun, Plawangan, Suwela, Labuan Haji), 1932 (Narmada, Teluk Ekas), 1934 (Segara Anak, Narmada, Sembalun, Labuan Haji, Suwela) 56 species.
-
3. HB Munaf & A Saim. 1980 (Pringgabaya, Keruak, Lombok Barat, Pujut, Suranadi, Aikmel). 42 species.
-
4. A Hanna. 1981 (Lombok Timur). One species.
-
5. Mudjiono & B Sudjoko. 1994 (Pantai Kuta, Tajung Aan, Pantai Gerupuk). 44 species.
-
6. Mudjiono. 1997 (Gili Trawangan, Gili Meno, Gili Air). 44 species.
-
7. W Kastoro & Maryoto. 1998 (Lombok Tengah, Pantai Kuta). Five species.
-
8. J Hylleberg. 1999 (Gili Trawangan, Gili Meno, Gili Air). 67 species.
-
9. W Kastoro, H Saito, K Hasegawa. 2000 (Lombok). 23 species.
-
10. P Prahoro & SP Anthony. 2000 (Batunampar, Teluk Ekas). 17 species.
-
11. DG Reid. 2002 (Pantai Kuta). One species.
-
12. E Yusron, P Darsono & P Widianwari. 2010 (Lombok Barat, Lombok Timur, Pantai Kuta). 24 species.
-
13. Y Apriyanti. 2013 (Sekotong, Lembar, Jerowaru). 28 species.
-
14. NR Isnaningsih. 2015 (Gangga, Keruak). 25 species.
-
15. M Zusron, CA Wibowo, A Langgeng, FM Firdausi, S Etfanti. 2015 (Sekotong). 29 species.
-
16. N Mujiono. 2016 (Sambelia, Praya Timur, Gili Lawang, Pujut, Gili Sulat, Senggigi). 31 species.
-
17. BP Gargely, JU Otani, T Hosoda, T Asami & J Harl. 2018 (Pujut). One species.
-
18. JR Parorrongan, F Zahida, IP Yuda. 2018 (Pantai Kuta). 27 species.
-
19. DA Candri, B Junaedah, H Ahyadi, Y Zamroni. 2018 (Lembar, Teluk Ekas, Sambelia). 47 species.
-
20. Athifah, MN Putri, SI Wahyudi, R Edy, IS Rohyani. 2019 (Taman Ayu). Seven species.
-
21. B Abdillah, Karnan, D Santoso. 2019 (Jerowaru). Nine species.
-
22. V Wardhani, SF Tania, BNA Pita, OY Rosada, NR Syafitri, N Amira, MA Akbar, Y Maulidan, SK Riandinata, DA Candri. 2019 (West and East Lombok). 47 species.
-
23. DA Candri, LH Sani, H Ahyadi, B Farista.
2020 (Jerowaru, Pantai Gerupuk). 34 species.
-
24. DA Candri, LH Sani, H Ahyadi, B Farista, A Virgota. 2020 (Bagek Kembar,West Lombok). 23 species.
Nomenclature of families is following Bouchet & Rocroi (2005), species names are validated following two websites: http://www.molluscabase.org and http://www.marinespecies.org. A species that is not listed in the examined literature is considered as New Record for the island.
RESULTS
Overall, 73 families belong to 292 species were documented from Lombok Island. To make them studied easier, they will be listed into four groups according to their habitat, i.e. freshwater, marine, terrestrial, and mangrove. The list can be seen below. Note: *stored in MZB, #type locality from Lombok, nr (new record), FW(freshwater), MG(mangrove), MR(marine), TR(terrestrial).
-
I. Ampullariidae
-
1. Pila scutata (Mousson, 1848) FW* : Pringgabaya, Keruak, Lombok Barat.
-
2. Pomacea canaliculata (Lamarck, 1822) FW : Taman Ayu.
-
II. Bithyniidae
-
3. Digoniostoma truncatum (Eydoux & Souleyet, 1852) FW* : Narmada, Pringgabaya.
-
III. Lymnaeidae
-
4. Radix rubiginosa (Michelin, 1831) FW* nr : Pringgabaya, Lombok Barat, Taman Ayu.
-
IV. Pachychilidae
-
5. Faunus ater (Linnaeus, 1758) FW MG* nr : Lembar, Sambelia.
-
V. Planorbidae
-
6. Gyraulus convexiusculus (Hutton, 1849) FW* :
Pringgabaya.
-
7. Indoplanorbis exustus (Deshayes, 1833) FW : Taman Ayu.
-
8. Planorbis elberti Haas, 1912 FW : Lombok
-
VI. Thiaridae
-
9. Melanoides tuberculata (Müller, 1774) FW MG* : Segara Anak, Pujut, Pringgabaya, Gangga, Sambelia, Taman Ayu.
-
10. Sermyla riqueti Grateloup, 1840 FW MG* : Labuan Haji, Gangga, Sambelia.
-
11. Stenomelania plicaria (Born, 1778) FW* : Gangga, Sambelia.
-
12. Stenomelania rustica (Mousson, 1857) FW* nr : Pringgabaya.
-
13. Tarebia granifera (Lamarck, 1816) FW* : Narmada, Pringgabaya, Lombok Barat, Suranadi, Gangga, Sambelia, Taman Ayu.
-
14. Thiara scabra (Müller, 1774) FW* : Pringgabaya, Lombok Barat, Taman Ayu.
-
VII. Viviparidae
-
15. Filopaludina javanica (von dem Busch, 1844) FW* : Narmada, Lombok Barat, Keruak, Taman Ayu.
-
VIII. Angariidae
-
16. Angaria delphinus (Linnaeus, 1758) MR : Gili Meno.
-
17. Angaria sp. MR*: Sekotong.
-
IX. Batillariidae
-
18. Batillaria zonalis (Bruguiere, 1792) MG MR* :
Lembar, Jerowaru, Keruak.
-
X. Buccinidae
-
19. Afer afer (Gmelin, 1791) MR : Pantai Kuta.
-
20. Buccinulum linea (Martyn, 1784) MR : Pantai Kuta.
-
21. Pollia undosa (Linnaeus, 1758) MR : Pantai Kuta, Tanjung Aan, Pantai Gerupuk.
-
XI. Bursidae
-
22. Bursa rhodostoma (Sowerby II, 1835) MR : Gili Meno.
-
XII. Calliostomatidae
-
23. Astele speciosa (Adams, 1855) MR : Teluk Ekas.
-
XIII. Cerithiidae
-
24. Cerithium coralium Kiener, 1841 MG MR*nr : Jerowaru, Lembar.
-
25. Cerithium punctatum Bruguière, 1792 MR : Pantai Kuta, Gili Meno, Gili Air, Lombok Barat, Lombok Timur, Batunampar, Teluk Ekas.
-
26. Cerithium rostratum Sowerby II, 1855 MR : Pantai Kuta, Lombok Barat, Lombok Timur.
-
27. Cerithium zonatum (Wood, 1828) MR*nr : Praya Timur.
-
28. Clypeomorus batillariaeformis Habe & Kosuge, 1966 MG MR : Lembar, Jerowaru, Praya Timur.
-
29. Clypeomorus pellucida (Hombron & Jacquinot, 1852) MG MR*nr : Jerowaru, Lembar.
-
30. Clypeomorus petrosa (Wood, 1828) MR :
Batunampar.
-
31. Pseudovertagus aluco (Linnaeus, 1758) MR* :
Lombok Barat, Pantai Kuta, Pantai Gerupuk.
-
32. Rhinoclavis sinensis (Gmelin, 1791) MR : Pantai Kuta, Tanjung Aan.
-
XIV. Chilodontidae
-
33. Euchelus atratus (Gmelin, 1791) MR*nr : Pantai Kuta, Pantai Gerupuk.
-
34. Granata elegans (Gray, 1847) MR : Pantai Kuta.
-
XV. Collumbelllidae
-
35. Amphissa versicolor Dall, 1871 MR : Pantai Kuta.
-
36. Euplica scripta (Lamarck, 1822) MR : Pantai Gerupuk, Pantai Kuta, Lombok Barat, Lombok Timur.
-
37. Mitrella scripta (Linnaeus, 1758) MR : Pantai Kuta.
-
38. Pardalinops testudinaria (Link, 1807) MR : Pantai Kuta, Teluk Ekas.
-
39. Pictocolumbella ocellata (Link, 1807) MR : Gili Trawangan, Gili Meno, Gili Air, Batunampar, Teluk Ekas, Pantai Kuta.
-
XVI. Conidae
-
40. Conus betulinus Linnaeus, 1758 MR : Pantai Kuta, Pantai Gerupuk.
-
41. Conus boeticus Reeve, 1844 MR : Gili Meno, Gili Trawangan, Gili Meno, Gili Air.
-
42. Conus capitaneus Linnaeus, 1758 MR : Pantai Kuta, Pantai Gerupuk.
-
43. Conus coffeae Gmelin, 1791 MR: Gili Trawangan, Gili Meno, Gili Air.
-
44. Conus coronatus Gmelin, 1791 MR : Gili Trawangan, Gili Meno, Gili Air.
-
45. Conus ebraeus Linnaeus, 1758 MR : Gili Trawangan, Pantai Kuta.
-
46. Conus eburneus Hwass in Bruguière, 1792 MR : Batunampar.
-
47. Conus emaciatus Reeve, 1849 MR : Gili Trawangan.
-
48. Conus figulinus Linnaeus, 1758 MR : Gili Trawangan, Gili Meno, Gili Air.
-
49. Conus flavidus Lamarck, 1810 MR : Gili Trawangan, Gili Meno, Gili Air.
-
50. Conus imperialis Linnaeus, 1758 MR : Gili Trawangan, Gili Meno, Gili Air.
-
51. Conus lividus Hwass in Bruguière, 1792 MR : Gili Trawangan, Gili Meno, Gili Air.
-
52. Conus malacanus Hwass in Bruguière, 1792 MR : Batunampar.
-
53. Conus marmoreus Linnaeus, 1758 MR : Pantai Gerupuk.
-
54. Conus miles Linnaeus, 1758 MR : Gili Trawangan.
-
55. Conus musicus Hwass in Bruguière, 1792 MR : Gili Trawangan, Gili Meno, Gili Air.
-
56. Conus planorbis Born, 1778 MR : Gili Trawangan, Gili Meno, Gili Air.
-
57. Conus rattus Hwass in Bruguière, 1792 MR : Gili Trawangan, Gili Meno, Gili Air.
-
58. Conus spectrum Linnaeus, 1758 MR : Teluk Ekas.
-
59. Conus striolatus Kiener, 1848 MR : Gili Trawangan, Gili Meno, Gili Air.
-
60. Conus suturatus Reeve, 1844 MR : Batunampar, Teluk Ekas.
-
61. Conus vexillum Gmelin, 1791 MR : Lombok Barat, Lombok Timur.
-
62. Conus virgo Linnaeus, 1758 MR : Gili Trawangan.
-
XVII. Costellariidae
-
63. Vexillum plicarium (Linnaeus, 1758) MR: Pantai Gerupuk.
-
64. Vexillum polygonum (Gmelin, 1791) MR : Batunampar.
-
65. Vexillum rugosum (Gmelin, 1791) MR : Pantai Gerupuk.
-
66. Vexillum vulpecula (Linnaeus, 1758) MR : Pantai Kuta, Lombok Barat.
-
67. Vexillum woldemarii (Kiener, 1838) MR : Batunampar.
-
XVIII. Cypraeidae
-
68. Arestorides argus (Linnaeus, 1758) MR : Pantai Kuta.
-
69. Bistolida kieneri (Hidalgo, 1906) MR : Pantai Kuta.
-
70. Cypraea tigris Linnaeus, 1758 MR* : Gili Lawang, Gili Trawangan.
-
71. Erosaria boivinii (Kiener, 1844) MR : Pantai Kuta.
-
72. Erronea caurica (Linnaeus, 1758) MR : Pantai Kuta.
-
73. Erronea errones (Linnaeus, 1758) MR * : Lombok Barat, Lombok Tengah, Pantai Gerupuk, Tanjung Aan, Pantai Kuta.
-
74. Luria isabella (Linnaeus, 1758) MR : Gili Meno.
-
75. Lyncina vitellus (Linnaeus, 1758) MR*nr : Lombok Tengah.
-
76. Lyncina lynx (Linnaeus, 1758) : Tanjung Aan, Pantai Gerupuk.
-
77. Mauritia arabica (Linnaeus, 1758) MR : Gili Trawangan.
-
78. Monetaria annulus (Linnaeus, 1758) MR* : Lombok Tengah, Gili Trawangan, Lombok Barat, Lombok Timur, Pantai Kuta.
-
79. Monetaria moneta (Linnaeus, 1758) MR : Lombok Tengah, Tanjung Aan, Pantai Gerupuk, Gili Trawangan, Gili Meno.
-
XIX. Fasciolariidae
-
80. Filifusus filamentosus (Röding, 1798) MR : Gili Trawangan.
-
XX. Fissurellidae
-
81. Montfortista panhi (Quoy & Gaimard, 1834) MR : Gili Trawangan, Gili Meno, Gili Air.
-
82. Scutus unguis (Linnaeus, 1758) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XXI. Haliotidae
-
83. Haliotis asinina Linnaeus, 1758 MR : Pantai Gerupuk.
-
84. Haliotis varia Linnaeus, 1758 MR : Pantai Gerupuk.
-
XXII. Littorinidae
-
85. Echinolittorina millegrana (Philippi, 1848) MR : Gili Trawangan, Gili Meno, Gili Air.
-
86. Echinolittorina pascua (Rosewater, 1970) MR : Gili Trawangan, Gili Meno, Gili Air.
-
87. Echinolittorina radiata (Souleyet in Eydoux & Souleyet, 1852) MR* : Pujut.
-
88. Echinolittorina sundaica (van Regteren Altena, 1945) MR : Gili Trawangan, Gili Meno, Gili Air.
-
89. Echinolittorina vidua (Gould, 1859) MR* : Kuta, Gili Trawangan, Gili Meno, Gili Air.
-
90. Littoraria carinifera (Menke, 1830) MG MR* : Lembar, Jerowaru, Praya Timur, Gili Trawangan, Gili Meno, Gili Air.
-
91. Littoraria intermedia (Philippi, 1846) MG MR : Gili Trawangan, Gili Meno, Gili Air.
-
92. Littoraria lutea (Philippi, 1847) MG MR : Gili Trawangan, Gili Meno, Gili Air.
-
93. Littoraria melanostoma (Gray, 1839) MG MR* : Lembar, Jerowaru.
-
94. Littoraria pallescens (Philippi, 1846) MG MR : Gili Trawangan, Gili Meno, Gili Air.
-
95. Littoraria scabra (Linnaeus, 1758) MG MR* : Lembar, Jerowaru, Gili Sulat, Praya Timur, Gili Lawang, Pujut, Sambelia, Tanjung Aan, Pantai Gerupuk, Gili Trawangan, Gili Meno, Gili Air.
-
96. Littoraria undulata (Gray, 1839) MG MR* : Pujut, Praya Timur, Gili Trawangan, Gili Meno, Gili Air.
-
97. Nodilittorina pyramidalis (Quoy & Gaimard, 1833) MR* : Pujut, Praya Timur.
-
98. Peasiella conoidalis (Pease, 1868) MR : Gili Trawangan, Gili Meno, Gili Air.
-
99. Peasiella fasciata Reid & Mak, 1998 MR : Gili Trawangan, Gili Meno, Gili Air.
-
100. Tectarius tectumpersicum (Linnaeus, 1758) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XXIII. Lottiidae
-
101. Liotinaria peronii (Kiener, 1838) MR : Gili Meno.
-
102. Patelloida saccharina (Linnaeus, 1758) MR* : Pujut, Gili Trawangan, Gili Meno, Gili Air.
-
103. Patelloida striata Quoy & Gaimard, 1834 MR* : Pujut, Gili Trawangan, Gili Meno, Gili Air.
-
XXIV. Mitridae
-
104. Mitra sp. MR* : Lembar.
-
105. Mitra stictica (Link, 1807) MR : Pantai Kuta.
-
106. Pterygia fenestrata (Lamarck, 1811) MR : Teluk Ekas.
-
107. Strigatella lugubris (Swainson, 1821) MR : Batunampar.
-
108. Strigatella pica (Dillwyn, 1817) MR : Pantai Kuta.
-
109. Strigatella retusa (Lamarck, 1811) MR : Gili Trawangan.
-
110. Strigatella telescopium (Reeve, 1844) MR : Batunampar.
-
111. Strigatella vexillum (Reeve, 1844) MR : Batunampar, Teluk Ekas.
-
XXV. Mitromorphidae
-
112. Mitromorpha columbellaria (Scacchi, 1836) MR : Gili Trawangan.
-
XXVI. Modulidae
-
113. Modulus tectum (Gmelin, 1791) MR*nr : Lombok Barat.
-
XXVII. Muricidae
-
114. Chicoreus brunneus (Link, 1807) MG MR : Tanjung Aan, Pantai Gerupuk.
-
115. Chicoreus capucinus (Lamarck, 1822) MG MR* : Jerowaru, Lembar, Sekotong, Gili Sulat.
-
116. Drupa morum Röding, 1798 MR : Gili Trawangan.
-
117. Drupella cornus (Röding, 1798) MR : Gili Trawangan.
-
118. Drupella margariticola (Broderip, 1833) MR * : Pujut, Pantai Kuta, Tanjung Aan, Pantai Gerupuk, Gili Trawangan, Gili Meno, Gili Air.
-
119. Drupina grossularia (Röding, 1798) MR : Gili Trawangan, Gili Meno, Gili Air.
-
120. Hexaplex cichoreum (Gmelin, 1791) MR*nr : Jerowaru.
-
121. Morula anaxares (Kiener, 1836) MR : Gili Trawangan, Gili Meno, Gili Air.
-
122. Muricodrupa fiscella (Gmelin, 1791) MR : Gili Trawangan, Gili Air.
-
123. Neothais marginatra (Blainville, 1832) MR : Gili Trawangan, Gili Meno, Gili Air.
-
124. Oppomorus purpureocinctus (Preston, 1909) MR : Gili Trawangan, Gili Meno, Gili Air.
-
125. Semiricinula fusca (Küster, 1862) MR : Gili Trawangan, Gili Meno, Gili Air.
-
126. Semiricinula muricoides (Blainville, 1832) MR : Gili Trawangan, Gili Meno, Gili Air.
-
127. Semiricinula squamosa (Pease, 1868) MR : Gili Trawangan, Gili Meno, Gili Air.
-
128. Semiricinula turbinoides (Blainville, 1832) MR : Gili Trawangan, Gili Meno, Gili Air.
-
129. Tenguella ceylonica (Dall, 1923) MR : Gili Trawangan, Gili Meno, Gili Air.
-
130. Tenguella granulata (Duclos, 1832) MR : Gili Trawangan, Gili Meno, Gili Air, Pantai Kuta.
-
131. Thalessa virgata (Dillwyn, 1817) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XXVIII. Nacellidae
-
132. Cellana testudinaria (Linnaeus, 1758) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XXIX. Nassariidae
-
133. Nassarius albescens (Dunker, 1846) MR : Lombok Barat, Lombok Timur.
-
134. Nassarius arcularia (Linnaeus, 1758) MR :
Batunampar.
-
135. Nassarius fissilabris (Adams, 1852) MR :
Batunampar, Teluk Ekas.
-
136. Nassarius gaudiosus (Hinds, 1844) MR : Pantai Kuta, Gili Trawangan, Gili Meno, Gili Air.
-
137. Nassarius globosus (Quoy & Gaimard, 1833) MR : Lombok Barat, Lombok Timur, Pantai Kuta.
-
138. Nassarius horridus MR (Dunker, 1847) :
Batunampar.
-
139. Nassarius pullus MR (Linnaeus, 1758) : Lombok Barat, Lombok Timur.
-
140. Nassarius sp. MG MR * : Jerowaru.
-
141. Nassarius venustus (Dunker, 1847) MR : Batunampar, Teluk Ekas.
-
XXX. Naticidae
-
142. Mammilla melanostoma (Gmelin, 1791) MR : Gili Trawangan, Gili Meno, Gili Air.
-
143. Natica fasciata (Röding, 1798) MR*nr : Lombok Barat.
-
144. Polinices mammilla (Linnaeus, 1758) MR* :
Lombok Barat, Pantai Kuta, Gili Trawangan, Gili Meno, Gili Air.
-
XXXI. Olividae
-
145. Oliva caerulea (Roding, 1798) MR : Pantai Kuta.
-
146. Oliva elegans Lamarck, 1811 MR : Gili Trawangan, Gili Meno, Gili Air.
-
147. Oliva oliva (Linnaeus, 1758) MR : Gili Trawangan, Gili Meno, Gili Air.
-
148. Oliva reticulata (Röding, 1798) MR : Pantai Kuta, Gili Trawangan, Gili Meno, Gili Air.
-
149. Oliva sericea (Röding, 1798) MR : Gili Trawangan, Gili Meno, Gili Air.
-
150. Oliva tricolor Lamarck, 1811 MR : Pantai Kuta.
-
152. Phasianella solida (Born, 1778) MR : Pantai Kuta.
-
XXXIV. Pisaniidae
-
153. Engina alveolata (Kiener, 1836) MR : Batunampar, Gili Trawangan.
-
154. Engina fusiformis Pease, 1865 MR : Pantai Kuta.
-
155. Engina maura (Sowerby, 1832) MR : Pantai Kuta.
-
156. Engina mendicaria (Linnaeus, 1758) MR : Pantai Kuta, Gili Trawangan.
-
157. Engina zonalis (Lamarck, 1822) MR : Pantai Kuta, Tanjung Aan.
-
XXXV. Planaxidae
-
158. Planaxis sulcatus (Born, 1778) MG MR : Jerowaru, Pujut, Praya Timur, Gili Trawangan, Gili Meno, Gili Air, Batunampar.
-
XXXVI. Pyramidellidae
-
159. Longchaeus eburneus (Laseron, 1959) MR : Batunampar.
-
160. Milda ventricosa (Guérin, 1831) MR : Lombok Timur, Lombok Barat, Batunampar.
-
161. Quirella humilis (Preston, 1905) MR : Plawangan.
-
162. Pyramidella maculosa Lamarck, 1822 MR : Lombok Barat.
-
XXXVII. Ranellidae
-
163. Gyrineum natator (Röding, 1798) MR*nr : Lombok Barat.
-
164. Monoplex pilearis (Linnaeus, 1758) MR : Pantai Gerupuk.
-
XXXVIII. Siphonariidae
-
165. Siphonaria normalis Gould, 1846 MR*nr : Pujut.
-
166. Siphonaria sirius Pilsbry, 1895 MR : Batunampar.
-
XXXIX. Strombidae
-
168. Canarium erythrinum (Dillwyn, 1817) MR : Gili Trawangan, Gili Meno, Gili Air.
-
169. Canarium labiatum (Röding, 1798) MR : Tanjung Aan, Pantai Gerupuk, Gili Meno.
-
170. Canarium mutabile (Swainson, 1821) MR : Gili Trawangan.
-
171. Conomurex luhuanus (Linnaeus, 1758) MR : Pantai Kuta, Tanjung Aan, Pantai Gerupuk, Gili Trawangan.
-
172. Euprotomus aurisdianae (Linnaeus, 1758) MR : Pantai Gerupuk, Gili Trawangan, Gili Meno, Gili Air.
-
173. Gibberulus gibberulus (Linnaeus, 1758) MR : Pantai Kuta.
-
174. Laevistrombus canarium (Linnaeus, 1758) MR : Lombk Timur.
-
175. Lambis lambis (Linnaeus, 1758) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XL. Tegulidae
-
176. Tectus fenestratus (Gmelin, 1791) MR : Pantai Kuta, Pantai Gerupuk, Gili Trawangan, Gili Meno, Gili Air, Lombok Barat, Lombok Timur.
-
177. Tectus niloticus (Linnaeus, 1767) MR : Pantai Gerupuk.
-
178. Tectus pyramis (Born, 1778) MR*nr : Gili Lawang.
-
XXXII. Patellidae
-
151. Scutellastra flexuosa (Quoy & Gaimard, 1834) MR : Gili Trawangan, Gili Meno, Gili Air.
-
XXXIII. Phasianellidae
-
XLI. Terebridae
-
179. Duplicaria spectabilis (Hinds, 1844) MR : Batunampar.
-
180. Hastula hectica (Linnaeus, 1758) MR : Pantai Kuta.
-
181. Oxymeris maculata (Linnaeus, 1758) MR : Pantai Kuta.
-
XLII. Tonnidae
-
182. Tonna canaliculata (Linnaeus, 1758) MR : Pantai Kuta, Pantai Gerupuk.
XLIII. Triviidae
-
183. Trivirostra oryza (Lamarck, 1810) MR : Pantai Kuta.
XLIV. Trochidae
-
184. Clanculus undatus (Lamarck, 1816) MR : Gili Trawangan, Gili Air.
-
185. Monodonta canalifera Lamarck, 1816 MR : Gili Trawangan, Gili Meno, Gili Air.
-
186. Monodonta labio (Linnaeus, 1758) MG MR* : Pujut, Sambelia, Pantai Kuta, Tanjung Aan, Pantai Gerupuk.
-
187. Pseudostomatella decolorata (Gould, 1848) MR : Gili Meno.
-
188. Stomatia phymotis Helbling, 1779 MR : Gili Trawangan.
-
189. Stomatella impertusa (Burrow, 1815) MR : Pantai Kuta
-
190. Trochus maculatus Linnaeus, 1758 MR : Lombok Barat, Pantai Kuta, Gili Trawangan, Gili Meno, Gili Air.
-
191. Trochus radiatus Gmelin, 1791 MR : Tanjung Aan, Pantai Gerupuk.
-
XLV. Turbinidae
-
192. Liotinaria peronii (Kiener, 1838) MR : Pantai Kuta.
-
193. Pomaulax gibberosus (Dillwyn, 1817) MR : Pantai Kuta.
-
194. Turbo argyrostomus Linnaeus, 1758 MR : Pantai Kuta.
-
195. Turbo chrysostomus Linnaeus, 1758 MR*nr :
Sekotong.
-
196. Turbo cidaris Gmelin, 1791 MR : Pantai Kuta.
-
197. Turbo petholatus Linnaeus, 1758 MR : Gili Trawangan, Gili Meno, Gili Air.
-
198. Turbo setosus Gmelin, 1791 MR* : Lombok Barat.
XLVI. Turbinellidae
-
199. Vasum ceramicum (Linnaeus, 1758) MR : Gili Trawangan.
-
200. Vasum turbinellus (Linnaeus, 1758) MR : Pantai Kuta.
XLVII. Velutinidae
-
201. Coriocella nigra Blainville, 1824 MR : Pantai Kuta.
XLVIII. Volutidae
-
202. Amoria zebra (Leach, 1814) MR : Pantai Kuta.
-
203. Cymbiola aulica (G. B. Sowerby I, 1825) MR : Pantai Kuta.
-
204. Cymbiola vespertilio (Linnaeus, 1758) MR : Pantai Kuta, Pantai Gerupuk, Gili Air, Lombok Barat.
XLIX. Ariophantidae
-
205. Elaphroconcha floresiana rufolineata Smith, 1898 TR# : Suwela, Sembalun.
-
206. Elaphroconcha fruhstorferi (Martens, 1896) TR# : Suwela, Sembalun.
-
207. Elaphroconcha internota Smith, 1898 TR# : Lombok.
-
208. Microcystina exigua (Moellendorff, 1897) TR : Plawangan, Suwela.
-
209. Lamprocystis sinica Möllendorff, 1885 TR : Lombok.
-
210. Parmarion everetti Collinge, 1897 TR# : Sembalun.
-
211. Parmarion intermedium Collinge, 1897 TR# : Suwela, Aikmel.
-
L. Camaenidae
-
212. Landouria smironensis (Mousson, 1849) TR : Suwela, Sembalun, Segara Anak.
-
213. Landouria rotatoria (Busch, 1842) TR : Plawangan, Segara Anak.
-
214. Planispira infarcta (Martens, 1896) TR# : Lombok.
-
215. Vulnus wallacei Pall-Gergely, Otani & Hosoda, 2017 TR : Pujut.
-
LI. Chronidae
-
216. Vitrinopsis fruhstorferi (Moellendorff, 1897) TR : Suwela.
-
LII. Cyclophoridae
-
217. Cyclotus politus (Sowerby I, 1843) TR : Lombok, Lepak.
-
218. Leptopoma vitreum (Lesson, 1830) TR*nr : Lombok Timur.
LIII. Diplommatinidae
-
219. Diplommatina lombockensis Smith, 1898 TR# : Lombok.
LIV. Dyakiidae
-
220. Asperitas trochus nemorensis (Müller, 1774) TR* : Gili Lawang, Suwela.
-
221. Sasakina oxyconus (Martens, 1896) TR# : Gunung Rinjani.
-
222. Sasakina perinsignis (Smith, 1898) TR# : Gunung Rinjani.
-
LV. Endodontidae
-
223. Philalanka nannophya Rensch, 1932 TR : Lombok.
LVI. Enidae
-
224. Ena batarae Rensch, 1930 TR# : Segara Anak.
LVII. Euconulidae
-
225. Coneuplecta collinae (Smith, 1898) TR# : Suwela.
-
226. Microcystis dyakana (Godwin-Austen, 1891) TR : Lombok.
-
227. Lamprocystis perglabra Smith, 1898 TR#*: Suwela.
-
228. Liardetia densetorta(Moellendorff, 1897) TR : Segara Anak.
-
229. Queridomus fimbriosus (Quadras & Moellendorff, 1894) TR : Lombok.
LVIII. Helicarionidae
-
230. Helicarion albellus Martens, 1867 TR : Lombok.
-
231. Helicarion lombockensis Rensch, 1830 TR# : Lombok.
LVIX. Pupinidae
-
232. Moulinsia floresiana (Smith, 1897) TR* : Suwela.
-
LX. Pyramidelliade
-
233. Quirella humilis (Preston, 1905) TR* : Plawangan, Segara Anak.
LXI. Subulinidae
-
234. Allopeas gracilis (Hutton, 1834) TR : Narmada.
-
235. Opeas soror Smith, 1898 TR# : Rinjani.
-
236. Paropeas achatinaceum (Pfeiffer, 1846) TR : Suwela, Sembalun.
-
237. Prosopeas alberti Haas, 1912 TR# : Suwela, Sembalun.
-
238. Prosopeas discernibilis (Martens, 1896) TR# : Sembalun.
-
239. Prosopeas lombockensis (Smith, 1898) TR# : Lombok.
LXII. Succineidae
-
240. Succinea javanica Schepman, 1912 TR : Segara Anak.
-
241. Succinea minuta Martens, 1867 TR : Lombok.
LXIII. Tornatellidae
-
242. Elasmias citreum (Smith, 1898) TR# : Lombok.
LXIV. Trochomorphidae
-
243. Videna bicolor Martens, 1864 TR : Suwela, Sembalun.
LXV. Truncatellidae
-
244. Truncatella guerinii Villa & Villa, 1841 TR : Teluk Ekas.
LXVII. Valloniidae
-
245. Pupisoma pulvisculum (Issel, 1874) TR : Lombok.
LXVII. Veronicellidae
-
246. Semperula maculata (Templeton, 1858) TR*nr : Narmada.
-
247. Semperula variegatula (Simroth, 1918) TR : Lombok
LXVIII. Assimineidae
-
248. Assiminea brevicula (Pfeiffer, 1855) MG* : Lembar, Keruak, Praya Timur.
LXIX. Ellobiidae
-
249. Carychium javanum Möllendorff, 1897 TR :
Lombok.
-
250. Cassidula aurisfelis (Bruguière, 1789) MG*:
Sambelia.
-
251. Cassidula nucleus (Gmelin, 1791) MG* : Gili
Lawang, Sambelia.
-
252. Cassidula sulculosa (Mousson, 1849) MG* : Gili Lawang, Sambelia.
-
253. Cassidula vespertilionis (Lesson, 1831) MG* : Gili Lawang.
-
254. Melampus sp. MG : Lembar, Sekotong.
LXX. Haminoeidae
-
255. Haminoea tenera (A. Adams, 1850) MG MR* : Praya Timur.
LXXI. Onchidiidae
-
256. Peronia verruculata (Cuvier, 1830) MG MR * : Praya Timur.
-
257. Platevindex sp. MG MR* : Praya Timur.
LXXII. Potamididae
-
258. Cerithidea obtusa (Lamarck, 1822) MG* : Lembar, Batunampar, Teluk Ekas.
-
259. Cerithidea quoyii (Hombron & Jacquinot, 1848) MG* : Lombok Tengah, Lembar, Keruak, Praya Timur.
-
260. Cerithideopsilla alata (Philippi, 1849) MG* :
Lembar, Keruak, Praya Timur.
-
261. Cerithideopsilla cingulata (Gmelin, 1791) MG* : Lembar, Jerowaru, Keruak, Praya Timur.
-
262. Telescopium telescopium (Linnaeus, 1758) MG* : Jerowaru, Gili Lawang.
-
263. Terebralia palustris (Linnaeus, 1767) MG* :
Lembar, Jerowaru, Keruak, Gili Sulat, Sambelia.
-
264. Terebralia sulcata (Born, 1778) MG* : Lembar, Jerowaru, Keruak, Gili Sulat, Sambelia, Gili Lawang.
LXXIII. Neritidae
-
265. Clithon bicolor (Récluz, 1843) FW*nr : Pringgabaya.
-
267. Clithon corona (Linnaeus, 1758) FW MG* : Sambelia, Pantai Kuta.
-
268. Clithon oualaniense (Lesson, 1831) FW MG* :
Jerowaru, Sambelia.
-
269. Nereina afra (Sowerby I, 1836) FW : Gili Air.
-
270. Neripteron bensoni (Récluz, 1850) MG MR : Gili Meno, Gili Air, Gili Trawangan.
-
271. Neripteron violaceum (Gmelin, 1791) FW MG : Pantai Kuta.
-
272. Nerita albicilla Linnaeus, 1758 MG MR : Tanjung Aan, Pantai Gerupuk, Gili Air, Gili Meno, Gili Trawangan, Pantai Kuta.
-
273. Nerita chamaeleon Linnaeus, 1758 MR : Gili Meno, Gili Air, Gili Trawangan.
-
274. Nerita costata Gmelin, 1791 MR* : Pujut, Tanjung Aan, Pantai Gerupuk, Gili Air, Gili Meno, Gili Trawangan, Pantai Kuta.
-
275. Nerita exuvia Linnaeus, 1758 MR* : Sekotong, Pujut, Gili Meno, Gili Air, Gili Trawangan.
-
276. Nerita georgina Recluz, 1841 MR : Gili Meno, Gili Air, Gili Trawangan.
-
277. Nerita histrio Linnaeus, 1758 MR : Gili Meno, Gili Air, Gili Trawangan.
-
278. Nerita litterata Gmelin, 1791 MR : Pantai Kuta.
-
279. Nerita ocellata Le Guillou, 1841 MR*nr : Pantai Senggigi, Pujut.
-
280. Nerita planospira Anton, 1838 MR* : Jerowaru, Lembar, Gili Lawang, Sambelia, Praya Timur, Gili Meno, Gili Air, Gili Trawangan.
-
281. Nerita plicata Linnaeus, 1758 MR*nr : Gili Air, Pantai Kuta, Pujut, Praya Timur, Gili Meno, Gili Air, Gili Trawangan.
-
282. Nerita polita Linnaeus, 1758 MR* : Gili Meno, Gili Air, Gili Trawangan, Pujut, Praya Timur.
-
283. Nerita signata Lamarck, 1822 MR* : Gili Air,
Jerowaru.
-
284. Nerita squamulata Guillou, 1841 MR*nr : Praya Timur.
-
285. Nerita undata Linnaeus, 1758 MR* : Gili Air,
Lembar, Jerowaru, Gili Lawang.
-
286. Neritina turrita (Gmelin, 1791) FW MG* : Sambelia.
-
287. Neritina variegata Lesson, 1831 FW MG* Sambelia.
-
288. Neritina waigiensis (Lesson, 1831) FW MG* :
Sambelia.
-
289. Neritodryas cornea Linnaeus, 1758 FW* : Sambelia.
-
290. Septaria porcellana (Linnaeus, 1758) FW*nr : Labuan Haji.
-
291. Smaragdia rangiana (Récluz, 1841) MR : Pantai Kuta.
-
292. Smaragdia souverbiana (Montrouzier in Souverbie & Montrouzier, 1863) MR : Gili Meno, Gili Air, Gili Trawangan.
DISCUSSION
The present study documents 73 families and 292 species of gastropoda. Eighteen species were described from their type locality in Lombok Island. Eighty-six species (29%) had their
specimen stored in MZB. Generally, a family of gastropods consisting of species adapted to a similar habitat. However, the results of this study indicate that there are several families whose members can live in more than one habitat. Carychium javanum is the only member of Ellobiidae that found live outside the mangrove. This species also found in the mountainous forest in West Java (Heryanto, 2017). Nine species from three families (Neritidae, Pachychilidae, Thiaridae) found in freshwater and mangrove habitat. Previous study found two species of Neritidae (Clithon corona, Neritina turrita) that live in both freshwater and mangrove habitat (Mujiono, 2016). Twenty species from nine families (Batillariidae, Cerithiidae, Littorinidae, Muricidae, Planaxidae, Trochidae, Haminoeidae, Onchidiidae, Neritidae) found in mangrove and marine habitat. Littorinidae consists of five genera and 16 species, only seven species (all from genus Littoraria) adapted to mangrove and marine habitat. Littoraria scabra and Littoraria carinifera are arboreal species that live on mangrove canopy (Mujiono, 2016). The other five Littoraria species are probably have a similar preference. Chicoreus is the only one genus of Muricidae that specialized to hunt their prey in mangrove (Tan, 2008; Mujiono, 2016). However, they also frequently found in open marine water (Rahmasari et al., 2015).
A family is considered as freshwater/marine/ terrestrial/mangrove if more than two-thirds of its species are live in a such habitat. The freshwater form consists of seven families (I-VII) and 15 species, eight of them occur in Pringgabaya. The marine form consists of 41 families (VIIIXLVIII) and 189 species, 90 of them occur in Gili Trawangan. The terrestrial form consists of 19 families (XLIX-LXVII) and 43 species, 13 of them occur in Suwela. The mangrove form consists of five families (LXVIII-LXXII) and 17 species, eight of them occur in Lembar. Meanwhile, Neritidae cannot be classified into the previous group because their member occupies a wide range of habitats such as marine, mangrove, and freshwater. They placed in a separate group because even in one genus, their species can be
adapted to two types of habitat (Clithon FW MG, Neripteron FW MG MR, Nerita MG MR, Neritina FW MG). There are five species which not previously recorded and considered as new records.
The data shows that Neritidae is the most diverse family with 28 species (9,5%) included in, followed by Conidae (23 species or 7,8%), Muricidae (18 species or 6,2%), and Littorinidae (16 species or 5,5%). Interestingly, the last three families are all marine forms. The freshwater form dominated by Thiaridae with six species. Terrestrial form dominated by two families, Ariophantidae and Subulinidae, each with seven species. Mangrove form dominated by Potamididae with seven species.
Figure 2 shows the diversity of gastropoda from 29 locations in Lombok Island. The average number of species of marine form is 42 (ranged 490 species), while for the non-marine form is 10 (ranged 1-22 species). The five most diverse locations were Gili Trawangan (90 species), Gili Meno (78 species), Gili Air (77 species), Pantai Kuta (65 species), and Pantai Gerupuk (29 species). Interestingly, they all are marine habitats. It is not surprising because the marine gastropoda was studied more extensively (Mudjiono & Sudjoko, 1994; Mudjiono, 1997; Hylleberg, 1999; Prahoro & Anthony, 2000; Reid, 2002; Yusron et al., 2010; Zusron et al., 2015; Parorrongan et al., 2018; Abdillah, 2019). Although they were extensively studied, however, there are 12 species which not previously recorded and considered as new records.
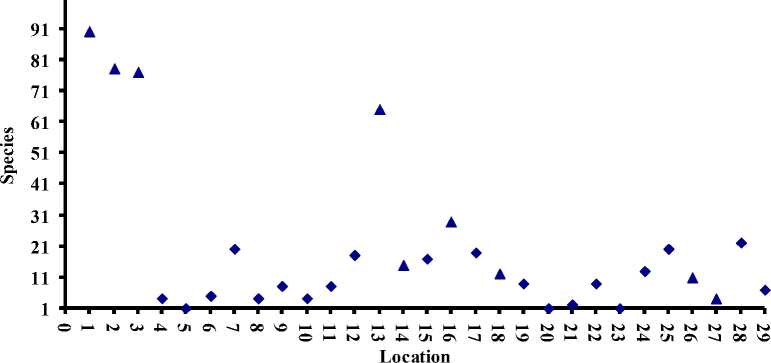
Figure 2. Cummulative of species number in each location. See Figure 1 for information of location’sname. ▲: marine form, ♦: non-marine form.
The first and last study on terrestrial gastropoda of Lombok were made by Smith (1898) and Páll-Gergely et al., (2018). Terrestrial gastropoda of Lombok Island was mainly studied by Rensch (1931, 1932). He spent two years (1927-1928) for collecting and studying land snail from Lombok Timur. He found 36 land snail species during his study, half of them are new to science. His discoveries make Lombok as an important place for studying biodiversity, especially terrestrial gastropoda. Rensch (1934) had summarized the diversity of terrestrial gastropoda from other island in the Lesser Sunda Islands. He listed 46 species in Bali, 37 species in Sumbawa, 54 species in Flores, and 43 species in Sumba. The data shows only two species which not previously recorded and considered as new records.
Freshwater gastropoda of Lombok Island was mainly studied by Rensch (1934) who listed the occurrence of 12 species. The present study by Athifah et al., (2019) listed only seven species from the Taman Ayu area. The diversity of freshwater gastropods in the Lesser Sunda Islands tends to be lower than their terrestrial relatives. Rensch (1934) listed 12 species in Bali, 15 species in Sumbawa, 14 species in Flores, and 17 species in Sumba. The data shows only three species which not previously recorded and considered as new records.
Mangrove gastropoda of Lombok Island was first studied by Isnaningsih (2015) who listed the occurrence of 25 species. Mujiono (2016) continued her study and adds 17 other species. The present studies by Candri et al., (2018, 2020, 2020) and Wardhani et al., (2020) are rather confusing. They listed more species than the previous studies. However, it also contains some marine families (Cerithiidae, Cymatiidae, Cypraeidae, Nassariidae, Strombidade, Trochidae, Turbinidae) which only the visitors in the mangrove forest. True mangrove species are Assiminidae, Ellobiidae, Onchidiidae, Potamididae and several species from Littorinidae, Muricidae, and Neritidae.
The gastropod diversity from the four habitat types in Lombok looks greatly different. This can be traced to the number of surveys or researches conducted. Marine species is dominant (189 species) because it was studied from ten locations and ten works of literature. It was followed by terrestrial species (43 species) that were studied from ten locations and five works of literature. Mangrove species (17 species) were studied from 12 locations and four works of literature. Freshwater species (15 species) were studied from ten locations and two works of literature.
From the present study, twenty-two new records for Lombok Island have been documented. It consists of three freshwater (Radix rubiginosa, Faunus ater, Stenomelania rustica), 12 marine (Cerithium coralium, Cerithium zonatum, Clypeomorus pellucida, Euchelus atratus, Lyncina vitellus, Modulus tectum, Hexaplex cichoreum, Natica fasciata, Gyrineum natator, Siphonaria normalis, Tectus pyramis, Turbo chrysostomus), two terrestrial species (Leptopoma vitreum, Semperula maculata), and five Neritidae (Clithon bicolor, Nerita ocellata, Nerita plicata, Nerita squamulata, Septaria porcellana). This finding is important for the biodiversity information from this island.
The species distributions were ranged from one to 12 from 29 observed locations. Littoraria scabra is the most widely distributed species which occurs in 12 locations. Nerita planospira occurs in eight locations. Seven species (Tarebia granifera, Cerithium punctatum, Drupella margariticola, Planaxis sulcatus, Tectus fenestratus, Nerita costata, Nerita plicata) occur in seven locations, while five species (Melanoides tuberculata, Pictocolumbella ocellata, Littoraria carinifera, Terebralia sulcata, Nerita albicilla) occur in six locations.
Gastropods are slow-moving animals. Aquatic species do not actively move through terrestrial habitats, terrestrial species do not actively disperse through water, those habitats act as the physical barriers. However, their distribution is beyond our expectations. Two methods of dispersal were known, active, and passive dispersal (Kramarenko, 2014). Gastropods are moving to search for food, a couple for mating, hiding from their enemies, or unsuitable physical condition (Chelazzi, 1991). Movement capabilities of some species have been recorded. For terrestrial species: Albinaria coerulea (Clausiliidae) moves along 750 cm during 30 hari (Giokas & Mylonas, 2004), Thersites mitchellae (Camaenidae) moves along 7.445 cm during 18 days (Parkyn et al., 2014), Lissachatina fulica (Subulinidae) moves along 1.672 cm during 3 days (Mujiono et al., 2019). For aquatic species: Pomacea paludosa (Ampullariidae)
moves along 600 cm/day (Darby et al., 2002), Neritina punctulata (Neritidae) moves along 7.400 cm/day (Pyron & Covich, 2003), Tarebia granifera (Thiaridae) moves along 5.760 cm/day (Snider & Gilliam, 2008).
Another way for gastropoda to be dispersed is by passive dispersal. Gastropoda does not actively move but being transported by another animal, by human, or by natural disaster. Insect and bird play important role in gastropod’s dispersal. Aerial insect such bee (Hymenoptera) frequently bring small arboreal gastropoda which accidentally attached to their legs while pollinating the flower. Water beetles (Coleoptera) also sometimes bring small aquatic gastropoda which accidentally attached to their outer wings while swimming. Because the body size of the vectors is small, they only can transport gastropoda locally. Bird with their ability to fly can transport gastropoda in much far distant, even to a different island. Arboreal and aquatic birds can bring larger gastropoda which accidentally attached to their legs or wings. Terrestrial gastropoda can be transported by migrating birds as far as 9000 km (Gittenberger et al., 2006). Natural disasters such as hurricanes can blow and bring both terrestrial and aquatic gastropods into another place in 28 km distance. While landslide can also bring some burrowing gastropoda down from the hill into the lower ground locally (Rees, 1965). Drift wood can be a vector for oceanic dispersal of some aquatic gastropoda (Kano et al., 2013).
The five most diverse locations are popular as the destination for marine tourism. There is a strong correlation between the location of tourism and the number of species found. Commonly, marine tourism is the favourite destination for most travelers. Some of them collecting the gastropods while swimming or diving. Gastropoda can be abundant in the littoral zone, the same area where peoples are swim or dive. It is good to inform the biodiversity of marine fauna (gastropoda) to the people using tourism as a tool. Some of gastropod’s families are known to be venomous, such as Cone (Conidae) and Auger (Terebridae) (Santhanam, 2017). All the members
of Conidae are found in the five locations above, while the Terebride are found in Pantai Kuta and Batunampar. Three species of Cone (Conus imperialis, Conus lividus, Conus marmoreus) are dangerous, even deadly. Their venom is capable of paralyzing people who accidentally got bitten. They maybe die within hours if they are not quickly treated. Three species of Auger (Duplicaria spectabilis, Hastula hectica, Oxymeris maculata) are also dangerous. However, their bites are less severe than the Cone (Santhanam, 2017). Another dangerous family is Murex (Muricidae). Their shell are spinous, some species have long and sharp spines. Peoples will get injured if they accidentally step on it. We can warn the peoples about these three dangerous gastropod’s families and tell them never to touch or catch to avoid being injured.
Despite their dangerous member of marine form, some families are known for their beautiful shells (Costellariidae, Cypraeidae, Mitridae, Olividae, Tegulidae, Volutidae) or being cultured for their shell and valuable meat (Haliotidae, Strombidae, Trochidae, Turbinidae) (Poutiers, 1998). Two species of Abalone (Haliotis asinina and Haliotis squamata) have been successfully cultured in Lombok (Kuncoro et al., 2013; Sinaga et al., 2015).
Based on the result of the present study, the diversity of mainland Lombok is less extensively studied compare with its satellite islands. Marine gastropods are mainly recorded from its five satellite islands. The composition of terrestrial gastropods is less diverse than Bali with 121 species (Vermeulen & Whitten, 1998) while freshwater gastropods also less diverse than Sumba with 23 species (Benthem Jutting, 1955). Therefore, more extensive studies are needed to uncover the diversity of gastropoda in Lombok Island more precisely. Island size may also contribute to animal diversity. Compared with the other four islands (Bali 5.780 km², Sumba 11.060 km², Flores 13.540 km², and Sumbawa 15.214 km²) in the Lessser Sunda islands, Lombok is the smallest (4.725 km²). Commonly, species diversity will increase following the island’s size, as known as the theory of island biogeography.
Because of the habitat in a small island (Lombok) is less varied than the larger one. Small islands can only support fewer species to life (Guo, 2015).
Two previous studies on the inventory of gastropoda species from other islands have been carried out. 875 species from 128 families listed from Singapore. It consist of 97 families and 723 marine species, five families and 66 mangrove species, 13 families and 50 terrestrial species, 13 families and 36 freshwater species (Tan & Woo, 2010). 243 species from 65 families listed from 20 islands in the Thousand Islands, Jakarta. It consist of 52 families and 214 marine species, nine families and 16 terrestrial species, three families and 13 freshwater species (Mujiono, 2015). Both studies show the same trends with the present study, marine species always dominant and freshwater species is less diverse than others.
CONCLUSION
This study summarizes the diversity of gastropods in Lombok. Seventy-three families and 292 species of gastropods were documented. Eighty six species (29%) of the specimens are kept at the Bogor Zoological Museum and 22 species are a new record for Lombok Island. The list is dominated by marine species (189 species or 65%), half of which are recorded from the three satellite islands. The diversity of mainland Lombok is still less extensively studied than its satellite islands. More extensive study is needed in the future.
ACKNOWLEDGEMENT
The author would like to thank Dr. H. Rustiami as the team coordinator and all the team members of The Lesser Sunda Expedition to Lombok Island 2015. Their kindness and help are greatly appreciated. Constructive advice from all the reviewers is also acknowledged.
REFERENCES
Abdillah B, Karnan, Santoso D. 2019. Struktur komunitas Mollusca (Gastropoda dan
Bivalvia) pada daerah intertidal di perairan pesisir Poton Bako Lombok Timur sebagai sumber belajar biologi. Jurnal Pijar MIPA 14(3): 208-216.
Ascui SH, Seow M. (Editors). 2005. DK Eyewitness Travel Guides Bali And Lombok. Dorling Kindersley Limited: London.
Athifah, Putri MN, Wahyudi SI, Edy R, Rohyani IS. 2019. Keanekaragaman Mollusca sebagai bioindikator kualitas perairan di kawasan TPA Kebon Kongok Lombok Barat. Jurnal Biologi Tropis 19(1): 54-60.
Bashar MA. 2018. Vision on biodiversity: ecotourism and biodiversity conservation in Bangladesh. Journal of Biodiversity Conservation and Bioresource Management 4(1): 1-10.
Benthem Jutting WSSV. 1955.
Susswassermollusken von Sumba.
Verhandlungen der Naturforschenden Gesellschaft in Basel 66: 49-69.
Bouchet P, Rocroi JP. 2005. Classification and nomenclátor of gastropod families. Malacologia 47(1-2): 1-397.
Candri DA, Junaedah B, Ahyadi H, Zamroni Y. 2018. Keanekaragaman moluska pada ekosistem mangrove di Pulau Lombok. BioWallacea Jurnal Ilmiah Ilmu Biologi 4(2): 88-93.
Candri DA, Sani LH, Ahyadi H, Farista B. 2020. Struktur komunitas Moluska di kawasan mangrove alami dan rehabilitasi pesisir selatan Pulau Lombok. Jurnal Biologi Tropis 20(1): 139-147.
Candri DA, Sani LH, Ahyadi H, Farista B, Virgota A. 2020. The composition of mollusks in mangrove ecosystem conservation area Bagek Kembar, West Lombok. IOP Conf. Series: Earth and Environmental Science 486: 1-7.
Chelazzi G. 1991. Eco-ethological aspects of homing behavior in molluscs. Ethological Ecology and Evolution 2(1): 11-26.
Darby PC, Bennetts RE, Miller SJ, Franklin Percival H. 2002. Movements of Florida
Apple Snails in relation to water levels and drying events. Wetlands 22: 489-498.
Giokas S, Mylonas M. 2004. Dispersal patterns and population structure of the land snail Albinaria coerulea (Pulmonata:
Clausiliidae). Journal of Molluscan Studies 70: 107-116.
Gittenberger E, Groenenberg DSJ, Kokshoorn B, Preece RC. 2006 Molecular trails from hitchhiking snails. Nature 439: 409.
Guo Q. 2015. Island Biogeography Theory: Emerging Patterns and Human Effects. In Reference Module in Earth Systems and Environmental Sciences. Elsevier.
Heryanto. 2017. Keragaman keong darat di hutan suksesi di Gunung Galunggung dan hutan tua Gunung Sawal, Jawa Barat. Zoo Indonesia 26(2): 59-69.
Hylleberg J. 1999. Molluscs from beaches on Bali, Lombok, Sumbawa, and Komodo Islands, Indonesia. Phuket Marine Biological Center Special Publication 19(2): 397-402.
Isnaningsih NR. 2015. Komunitas moluska di ekosistem mangrove Pulau Lombok. Oseanologi dan Limnologi di Indonesia 41(2): 121-131.
Kano Y, Fukumori H, Brenzinger B, Warén A. 2013. Driftwood as a vector for the oceanic dispersal of estuarine gastropods (neritidae) and an evolutionary pathway to the sunken-wood community. Journal of Molluscan Studies 79: 378-382.
Kastoro W, Saito H, Hasegawa K. 2000. Phylum Mollusca. In : Matsura K, Sumadhiharga OK, Tsukamoto K. (eds). Field Guide to Lombok Island: Identification Guide to Marine Organisms in Seagrass Beds of Lombok Island, Indonesia. Ocean Research Institute: Tokyo.
Kitchener DJ, Boeadi, Charlton L, Maharadatunkamsi. 1990. The wild Mammals of Lombok Island: Nusa
Tenggara, Indonesia: systematics and natural history. Records of the Western Australian Museum Supplement No. 33: 1-129.
Kramarenko SS. 2014. Active and passive migration of terrestrial mollusks: an
overview. Ruthenica 24(1): 1-14.
Kuncoro A, Sudaryono A, Sujangka A, Setyabudi H, Suminto. 2013. Pengaruh pemberian pakan buatan dengan sumber protein yang berbeda terhadap efisiensi pakan, laju pertumbuhan, dan kelulushidupan benih abalone hybrid. Journal of Aquaculture Management and Technology 2(3): 56-63.
MoluscaBase. 2020. Accessed via
Monk KA, Fretes YD, Lilley GR. 2000. Ekologi Nusa Tenggara dan Maluku. Seri Ekologi Indonesia. Buku V. Prenhallindo: Jakarta.
Mudjiono, Sudjoko B. 1994. Fauna moluska padang lamun dari pantai Pulau Lombok Selatan. In: Kiswara W, Moosa MK, Hutomo M (eds) Struktur Komunitas Biologi Padang Lamun di Pantai Selatan Lombok dan Kondisi Lingkungannya. Puslitbang Oseanografi LIPI: Jakarta.
Mudjiono. 1997. Telaah struktur komunitas moluska di rataan terumbu Gili Trawangan, Gili Meno, dan Gili Air, Lombok Barat (NTB). Prosiding Seminar Nasional Pengelolaan Terumbu Karang: 96-103.
Mujiono N.. 2015. Gastropoda dari Kepulauan Seribu, Jakarta berdasarkan koleksi spesimen Museum Zoologi Bogor. Prosiding Seminar Nasional Masyarakat Biodiversitas Indonesia 1(8): 1771-1784.
Mujiono N. 2016. Gastropoda mangrove dari Pulau Lombok, Nusa Tenggara Barat. Oseanologi dan Limnologi di Indonesia 1(3): 39-50.
Mujiono N, Mardliyah ZR, Putri VW, Putri AE, Raffiudin R. 2019. Perilaku lokomosi, homing, dan kawin pada Bekicot (Lissachatina fulica Bowdich, 1822). Zoo Indonesia 28(1): 21-32.
Parorrongan JR, Zahida F, Yuda IP. 2018. Keanekaragaman dan kelimpahan
Gastropoda di Pantai Seger, Lombok Tengah. Biota 3(2): 79-86.
Páll-Gergely B, Otani JU, Hosoda T, Asami T, Harl J. 2018. A new species of Camaenidae (Gastropoda, Pulmonata) from Nusa Penida and Lombok Islands, Indonesia: novelty in a well-known fauna. Molluscan
Research 38(1): 41-49.
Parkyn J, Brooks L, Newell D. 2014. Habitat use and movement patterns of the endangered land snail Thersites mitchellae (Cox, 1864) (Camaenidae). Malacologia 57(2): 295-307.
Prahoro P, Sisco AP. 2000. Keanekaragaman jenis keong (Gastropoda) di Pantai Ujung Kelor, Batunampar, dan Gunung Linus Teluk Ekas, Lombok Timur (NTB). Jurnal Penelitian Perikanan lndonesia 6(2): 84-91.
Poutiers J. 1998. Gastropods. In: Carpenter KE & Niem VH (eds). FAO Species Identification Guide for Fishery Purpose. The Living Marine Resources of the Western Central Pacific. Volume 1. Seaweeds, Corals,
Bivalves, and Gastropods. FAO: Rome.
Pyron M, Covich AP. 2003. Migration patterns, densities, and growth of Neritina punctulata snails in Rio Espiritu Santo and Rio Mameyes, northeastern Puerto Rico. Caribbean Journal of Science 39: 338-347.
Rahmasari T, Purnomo T, Ambarwati R. 2015. Keanekaragaman dan kelimpahan
gastropoda di Pantai Selatan Kabupaten Pamekasan, Madura. Biosaintifika 7(1): 814.
Rees WJ. 1965. The aerial dispersal of Mollusca. Proceedings of the Malacological Society of London 36: 269-282.
Rensch B. 1931. Die molluskenfauna der Kleinen Sunda-Inseln Bali, Lombok, Sumbawa, Flores und Sumba. I. Zoologische
Jahrbücher Abteilung für Systematik,
Ökologie und Geographie der Tiere 61: 361396.
Rensch B. 1932. Die molluskenfauna der Kleinen Sunda-Inseln Bali, Lombok, Sumbawa, Flores und Sumba. II. Zoologische
Jahrbücher Abteilung für Systematik,
Ökologie und Geographie der Tiere 63: 1130.
Rensch, B. 1934. Die molluskenfauna der Kleinen Sunda-Inseln Bali, Lombok, Sumbawa, Flores und Sumba. III. Zoologische Jahrbücher Abteilung für Systematik, Ökologie und Geographie der Tiere 65: 389-422.
Rosenberg G. 2014. A new critical estimate of named species-level diversity of the recent Mollusca. American Malacological Bulletin 32(2): 308-322.
Rustiami H, Windadri FI, Tihurua EF, Sunarti S, Sulistyaningsih LD, Dewi, Rugayah, Rahayu M, Mansur M, Mahyuni R. 2020. Checklist Flora of Lombok. Herbarium Bogoriense, Research Center for Biology, Indonesian Institute of Sciences: Bogor.
Santhanam R. 2017. Biology and Ecology of Venomous Marine Snails. Apple Academic Press: New Jersey.
Sinaga DS, Melki, Setyono DED. 2015. Studi pertumbuhan abalon tropis (Haliotis asinina) dengan pemberian pakan buatan yang berbeda. Maspari Journal 7(1): 21-28.
Smith EA. 1898. A list of the land-shells of the island of Lombock, with descriptions of new species. Proceedings of the Malacological Society of London 3: 26-33.
Snider SB, Gilliam JF. 2008. Movement ecology: size-specific behavioral response of an invasive snail to food availability. Ecology 89:1961-1971.
Strong EE, Gargominy O, Ponder WF, Bouchet P. 2008. Global diversity of gastropods
(Gastropoda; Mollusca) in freshwater.
Hydrobiologia 595:149-166.
Tan KS. 2008. Mudflat predation on bivalves and gastropods by Chicoreus capucinus (Neogastropoda: Muricidae) at Kungkrabaen Bay, Gulf of Thailand. The Raffles Bulletin of Zoology Supplement No 18:235-245.
Tan SK, Woo HPM. 2010. A Preliminary Checklist of the Molluscs of Singapore. Raffles Museum of Biodiversity Research, National University of Singapore: Singapore.
Vermeullen JJ, Whitten AJ. 1998. Fauna Malesiana Guide to the Land Snail of Bali. Backhuys Publishers: Leiden.
Wardhani V, Tania SF, Pita BNA, Rosada OY, Syafitri NR, Amira N, Akbar MA, Maulidan Y, Riandinata SK, Candri DA. 2019. Molluscs from mangrove ecosystem in West Lombok and East Lombok. Proceedings of 2nd Internatonal Conference on Bioscience, Biotechnology and Biometrics (ICBBB 2019): 1-8.
World Register of Marine Species. 2020.
Accessed via http://www.marinespecies.org
Yusron E. 2010. Penelitian Kajian Diversitas Biota Laut di Perairan Lombok dan Sekitarnya, Nusa Tenggara Barat. Laporan Akhir Program Insentif Riset Peneliti Dan Perekayasa LIPI Tahun 2010. Puslit Oseanografi LIPI: Jakarta.
Zusron M, Wibowo CA, Langgeng A, Firdausi FM, Etfanti S. 2015. Biodiversity of Mollusks at Ela-Ela Beach, Sekotong Lombok Barat Indonesia. KnE Life Sciences 2: 574-578.
86
Discussion and feedback