PHENOLOGY, POLLINATION AND SEED PRODUCTION OF Millettia pinnata IN KUNUNURRA, NORTHERN WESTERN AUSTRALIA
on
JURNAL BIOLOGI XVIII (1) : 19 - 23
ISSN : 1410-5292
PHENOLOGY, POLLINATION AND SEED PRODUCTION OF Millettia pinnata IN KUNUNURRA, NORTHERN WESTERN AUSTRALIA
Ni Luh Arpiwi1,2*, Guijun Yan1, Elizabeth L Barbour1,3, Julie A Plummer1
1School of Plant Biology, Faculty of Natural and Agricultural Sciences The University of Western Australia, 35 Stirling Hwy Crawley, WA 6009, Australia 2Biology Department, Faculty of Math and Natural Sciences, Udayana University, Bali, Indonesia.
3Forest Products Commission of Western Australia, 117 Great Eastern Highway Rivervale, WA 6103, Australia.
Email: niluharpiwi@ymail.com
INTISARI
Millettia pinnata L. Panigrahi atau Pongamia pinnata L. Piere adalah tumbuhan legum yang menghasilkan biji dengan kandungan minyak yang cocok untuk biodiesel. Fenologi dan polinasi pada tanaman ini dipantau dan dimanipulasi untuk meningkatkan pembentukan biji dan hasil panen. Musim berbunga di Kununurra berlangsung selama satu bulan dari awal Oktober dan pembungaan terjadi tidak bersamaan baik pada satu tanaman maupun di antara tanaman yang berbeda. Dua spesies lebah lokal sebagai polinator, Megachile sp., dan Nomia sp., memiliki tingkat kunjungan yang sangat rendah (6-13 kunjungan) dengan puncak waktu kunjungan dari jam 9.00 sampai jam 10.00 dan periode aktivitas mencari makan yang pendek (dari jam 6.00 sampai 12.00). Hal ini mungkin terjadi karena sedikitnya volume nektar yang dihasilkan (1.0 ± 0.04 µl) dan tingginya temperatur di Kununurra. Viabilitas polen yang tinggi (85 ± 3%) menurun tajam menjadi 10 ± 3% dengan penyimpanan selama satu tahun pada suhu 4oC, tetapi hanya sedikit menurun menjadi 62 ± 3% pada suhu penyimpanan -20oC dan -80oC. Kunjungan lebah madu (Apis mellifera) meningkatkan hasil panen biji dari 296 menjadi 4.981 g/pohon, tetapi hasil panen ini masih rendah dan sangat bervariasi.
Kata kunci: morfologi bunga, fenologi, lebah madu, Millettia pinnata, viabilitas polen, hasil panen biji
ABSTRACT
Millettia pinnata L. Panigrahi syn. Pongamia pinnata L. Pierre is a leguminous tree, which produces seed oil suitable for biodiesel. Phenology and pollination were monitored and manipulated to increase seed set and yield. Flowering time in Kununurra occurred for one month from early October and flowering was asynchronous within and between trees. Two legitimate, native bee pollinators, Megachile sp. and Nomia sp., had very low visitation rates (6 and 13 visits) during the peak period from 09.00 – 10.00 and short foraging activity (from 06.00 – 12.00). This was possibly due to the small nectar reward (1.0 ± 0.04 µl) and high summer temperature in Kununurra. High pollen viability (85 ± 3%) decreased substantially following storage for 1 year at 4oC to 10 ± 3%, but only slightly to 62 ± 3% at either -20 oC or -80 oC. Introduced honey bees (Apis mellifera) increased seed yield from 296 to 4981 g/tree, but it remained relatively low and highly variable.
Keywords: flower morphology, phenology, honey bee, Millettia pinnata, pollen viability, seed yield
INTRODUCTION
Millettia pinnata (L). Panigrahi syn Pongamia pinnata L. (Pierre) is one of the few leguminous trees that produce seeds with high oil content, therefore, it is useful for biodiesel. This new tree crop has multiple uses, including many by-products and extracts from the seed, which could improve profitability of farming systems and commercial plantations (Scott et al., 2008). Information gathered from natural and ornamental trees indicates this crop has substantial potential for successful biodiesel production (Murphy et al., 2012). However, little is known about yield of M. pinnata in plantation environments, in particular the impact of factors such as flowering, pollination and pod set on seed yield. These were investigated in mature trees in Kununurra Western Australia, which are in the oldest plantations in Australia.
Millettia pinnata originates from India (Sujatha et al., 2008) and across Asia into the Pacific (Morton,
1990; Scott et al., 2008). Flowering time varies with individual trees across locations (Dhillon et al., 2009; Raju and Rao, 2006), but the diversity of flowering time is poorly understood. The monsoonal tropics of northern Australia have a distinct hot wet and cooler dry season. Most rain falls from November till March with an annual rainfall of 800 mm (Bureau of Meteorology, 2010). Mean maximum monthly temperatures range from 30°C in the coolest month of July to 39°C in November, mean monthly minimum temperatures range from 15°C in July to 26°C in December and phenology will need to be determined under these climatic conditions. Flowers take 11 months to develop into mature seeds (Arpiwi et al., 2012). Hence, reproductive phenology requires investigation.
Millettia pinnata belongs to Family Fabaceae, which has unique floral architecture (Aronne et al., 2012). Bisexual, zygomorphic flowers have one large standard petal, two light-purple wing petals and two white keel
petals. Standard petals have a hook-like structure at the base to hold nectar. Anthers and stigma are enclosed but separated in the boat-shaped keel petals (Raju and Rao, 2006). These require tripping agents to bring about pollination and the most common, legitimate pollinators are bees (Galloni et al., 2007). Legitimate pollinators will need to be identified and managed for M. pinnata plantations.
Pollen viability can be limited across-pollination. Pollen viability can be reduced by temperature extremes in the field (Kakani et al., 2005; Austin et al., 1996) or prolonged low temperatures suitable for storage (Astarini et al., 1999). Superior genotypes of M. pinnata has been sought (Arpiwi et al., 2012) and controlled cross-pollination required for breeding was assisted by pollen storage. Viable pollen can be distinguished from dead pollen using fluorescence microscopy (George at al., 2009).
The aims of this study were to investigate phenology, pollination and the rule of native and introduced pollinators on seed set and seed yield. Pollen viability under different time and temperatures were also studied.
MATERIALS AND METHODS
Flowering penology and floral traits
Plants (20) were randomly selected within the Forest Products Commission collection of Kununurra (Lat 15° 46´ S, Long 128° 44´ E) in tropical, northern Western Australia. Flowering phenology was observed from the beginning (first week of October 2012) until the end (first week of November 2012) of the flowering season on 20 selected trees. Flower characteristics, including length of inflorescence and number of flowers and buds in an inflorescence, were examined on 30 inflorescences from three trees. Timing and duration of flower opening were noted by removing open flowers from an inflorescence to avoid recounting. Time of dehiscence, when anthers began to disperse pollen, was measured on 20 mature buds (one day before flower opening) in hourly interval from 06.00 to 10.00. Buds were opened, and anthers removed and observed over paper using a 10x hand lens. Stigma receptivity was measured in mature buds (20) and open flowers (20) at 07.00, 12.00 and 16.00 using Peroxtesmo Ko indicator paper (Macherey-Nagel GmbH & Co, Germany) according to the method by Dafni and Maues (1998). Nectar was collected from bagged inflorescences before flower opening to avoid insect removal of nectar. When flowers opened bags were removed and 30 flowers from 3 trees and nectar was collected using a micropipette and the volume measured.
Flower visitors
Flower visitors to three selected trees were observed. Their activities, including mode of approach, forage collected and contact with anther and stigma were recorded. The number of visits by each visitor was monitored at hourly intervals for 30 min from 06.00 to 17.00. Visitors were captured and identified in the Entomology Section of the Western Australian Museum.
Pollen viability
Pollen viability and storage potential were examined using a method by George et al., (2009). Nine inflorescences were harvested from three trees and air dried over paper for 4 h. Anthers were cut from flowers, bulked and air dried (48 h) in a desiccator containing silica gel. Samples were stored at 4oC, -20oC or -80oC for 0, 90, 180, 270 and 360 days. Following storage, anthers were suspended in 1 ml of 20% sucrose and agitated to release pollen, then left for 10 min to hydrate pollen grains. A microscope slide was prepared with a drop of 1% flourescein diacetate in acetone, evaporated and then a drop of pollen suspension was added. Staining was viewed under a Zeiss Axioplan fluorescence microscope MC 80 with a blue filter combination. Viable pollens fluoresced bright yellow-green while non-viable pollens were opaque green or did not fluoresce. Viability was calculated as percentage of fluorescent pollen over total pollen grains.
Pod and seed yields
Pod and seed were harvested in two consecutive years to study the influence of introduced honey bees on pod and seed set. Five hives were introduced to a M. pinnata plot containing 10 year-old trees planted into laser-leveled irrigated plots at 3.6 m x 6 m spacing. Bee hives were placed 6 m apart from the trees early in October 2008 during flowering time. Pods were hand harvested from 20 trees in September 2008 and 2009 respectively and weighed. These will give yields comparison with and without introduced honey bees.
Data analysis
Mean and standard errors of floral traits were calculated using Microsoft Excel. Pollen viability at different storage temperatures and durations were subjected to analysis of variance using Genstat 14th (VSN International, UK). Mean and standard error of mean were determined.
RESULTS
Flowering phenology and floral traits
Flowering in Kununurra commenced in the first week of October and lasted until the first week of November and it varied within a tree and between trees. Within a tree new flowers bloomed few days after the first bloomed, while between trees the difference could be up to eighteen days. Inflorescences were 16.5 ± 0.3 cm long with 78 ± 1.2 flowers (Figure 1). Flowers opened from 06.00 to 10.00 in the morning and they remained open until 17.00. Each flower opened for one

Figure 1. Inflorescences of Mil-lettia pinnata
day only, there were 10-15 flowers open per inflorescence per day, and flowers opened in acropetal order (from base to tip) within the inflorescence. All flowers within an inflorescence opened within 9 ± 0.3 days and the ovary began to swell into a pod 8 ± 0.5 days after the flower opened. Anthers started to dehisce at the mature bud stage, one day before flower opening. Stigmas were receptive in mature buds just before opening and in open flowers from 06.00 to 17.00. Nectar volume was 1.0 ± 0.04 µl per flower and it was available from 06.00 to 11.00 with only a negligible amount detected after this time.
Flower visitors
Three species of local insects visited flowers, and these were native bees, Megachile sp. and Nomia sp., and a wasp Polistes sp. (Figure 2). During the monitoring period from 06.00 to 18.00 more visits were recorded of Apis mellifera than Megachile sp., followed by Nomia sp and Polistes sp. (Figure 3). Foraging activities started at 06.00 and most flower visitors had their peak activity of 6-12 visits between 09.00 and 10.00 on the bright, sunny days while Apis mellifera made about 100 visits at this time. Flower visits began to decrease after 10.00 and there were no visits after 12.00.
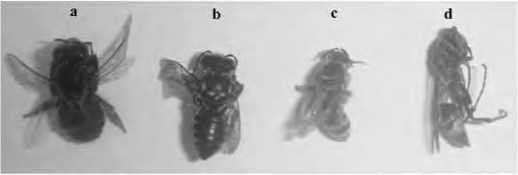
Figure 2. Flower visitors to Millettia pinnata in plantations at Kununurra: a) Apis mellifera, b) Megachile sp., c) Nomia sp. and d) Polistes sp.
Apis mellifera, Megachile sp. and Nomia sp. visited both mature buds and open flowers, where keel petals provided a platform for landing. These bees tripped the keel petals from the front without side-working and moved toward the base of the standard petal for nectar collection. Their weight freed stigma and stamens and these contacted with ventral side of the bees carrying pollens from previously visited flowers, then cloud of pollens were ejected explosively dusting the ventral sides of the bees. Apis mellifera collected pollen and carried it in the pollen baskets on their hind legs, but Megachile sp. and Nomia sp carried pollen in the underside of abdomen. The wasp (Polistes sp.) foraged on petals for nectar droplets with side-working, but without body contact with the stigma. Wasps foraged for nectar by perforating the calyx and very rarely tripped the keel. There was a maximum of seven wasp visits at the peak time between 9.00 and 10.00.
Pollen viability
Pollen viability was influenced by storage temperature and duration (P<0.001, Figure 4). Viability of fresh pollen was 85 ± 3%. Within three months of storage at 4oC viability decreased substantially to 58 ± 4% and this
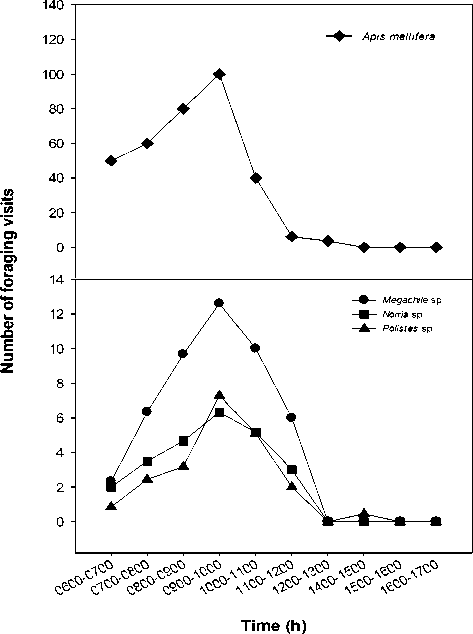
Figure 3. Activities of flower visitors: Apis mellifera (a), and Megachile sp., Nomia sp. and Polistes sp. (b) from 06.00 to 17.00 on Millettia pinnata grown in plantations at Kununurra
further decreased to a third after nine months and to 10 ± 4% after a year. At lower temperatures viability declined at a slower rate and it did not change within 90 days but reduced to 67 ± 4% after 180 days (-20oC) and 270 days (-80oC ). The viability of pollen stored for a year decreased slightly to 62 ± 3% at both low temperatures.
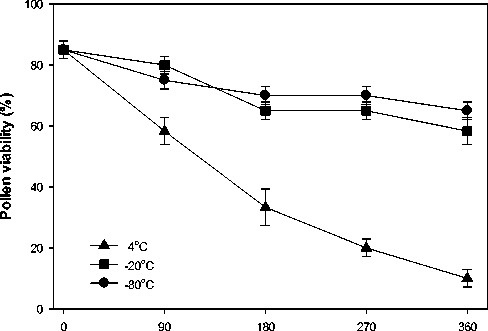
Storage duration (days)
Figure 4. Millettia pinnata pollen viability after storage for 0, 90, 180, 270 and 360 days at 4oC, -20oC and -80oC. Values are mean ± SE, n =3
Seed and pod yield
Yields of 20 randomly selected trees were highly variable between trees (P<0.001) both with and without
Table 1. Pod and seed yield (g) per tree with and without bee hives containing honey bee (Apis mellifera). SE = Standard error
|
Tree # |
Yield/tree (g) without bees |
Yield/tree (g) with bees | ||
|
Pod |
Seed |
Pod |
Seed | |
|
1 |
385 |
160 |
365 |
150 |
|
2 |
380 |
170 |
14855 |
5950 |
|
3 |
0 |
0 |
9100 |
3700 |
|
4 |
0 |
0 |
265 |
110 |
|
5 |
0 |
0 |
1200 |
470 |
|
6 |
0 |
0 |
1220 |
490 |
|
7 |
495 |
180 |
1180 |
480 |
|
8 |
0 |
0 |
295 |
110 |
|
9 |
0 |
0 |
2000 |
850 |
|
10 |
3500 |
1450 |
10905 |
4300 |
|
11 |
5000 |
2100 |
60335 |
24200 |
|
12 |
125 |
50 |
285 |
100 |
|
13 |
0 |
0 |
10715 |
4200 |
|
14 |
650 |
250 |
1515 |
600 |
|
15 |
1060 |
500 |
90905 |
36400 |
|
16 |
165 |
60 |
1670 |
700 |
|
17 |
445 |
180 |
38240 |
15300 |
|
18 |
665 |
300 |
2445 |
950 |
|
19 |
1375 |
500 |
265 |
110 |
|
20 |
52 |
20 |
1105 |
450 |
|
Mean |
715 |
296 |
12443 |
4981 |
|
SE |
160 |
66 |
2782 |
1114 |
bees (Table 1). In 2008 without bee hives, seven trees did not produce any pods, and the remaining trees had a pod yield of 715 g/tree and seed yield of 296 g/tree. In 2009 after the introduction of bee hives all 20 trees produced pods with 12,443 g pods/tree and seed yield of 4,981 g/tree. It should be noted that trees were also one year older.
DISCUSSION
With some variations between trees flowering in Kununurra commenced in the first week of October and ended during the first week of November. There was considerable overlap in the duration of flowering between trees, even though the first flowers in inflorescences opened at different times within a tree. In some tree crops, such as apricot, asynchrony of flowering reduces fruit set (Austin et al., 1998) but the limited asynchrony in M. pinnata should not limit cross pollination as there were some flowers open on several trees at any one time.
Native pollinators were very rare in Kununurra, and only two species of legitimate pollinators were observed on M. pinnata trees, namely two bees Megachile sp. and Nomia sp. Both Megachile sp. and Nomia sp. triggered the pollen release mechanism when they landed on enclosed keel petals and depressed these petals while seeking nectar at the base on standard petal. The bees grasped the flower and their abdomens always made contact with the stigma while foraging allowing for transfer of pollen from previously visited flowers. Local bees were legitimate pollinators, who probably transferred pollen resulting in cross fertilizations and successful seed set. A single species of wasp, Polistes sp. also visited flowers, but this insect was an illegitimate pollinator and it did not trigger the pollen release mechanism.
Insects, foraged on flowers, reached a peak activity between 09.00 and 10.00 with 6-12 visits. The low foraging activity was possibly due to the low volume of
nectar and high daytime temperatures in Kununurra during flowering. High diversity, abundance and activity of pollinators are usually related to copious nectar availability (Bhattacharya, 2004). In Kununurra only 1 µl of nectar was available per flower from 06.00 to 11.00 and negligible amounts were detected thereafter. This may be due to the extreme temperatures in Kununurra with the highest maximum of 40.8oC in October which could lead to evaporation of nectar (Herrera, 1990) and reduced insect activity.
Overall, the activity of introduced honey bees was much greater than native bees. Whilst there are 800 bee species in Western Australia, most of them are solitary (Houston, 2011). Solitary bees are more specialized pollinating particular species of flowers. They have shorter lifespans and have morphological and behavioral traits that fit the host plants but they are often ineffective pollinators for non-host plants. In contrast, honey bees live in hives of thousands of individuals (Austin et al., 1996), colonies live year-around and they are generalists and rely on many flower species for survival (Westerkamps and Gottsberger, 2000).
Pollen viability was high at 85% and this should have been adequate for cross-pollination and seed set. The number and viability of pollen grain deposited onto the stigma was not a limiting factor for seed set in M. pinnata (Arathi et al., 1999). Pollen viability usually remains high over the short periods of time required for dispersal and cross-pollination (Astarini et al., 1999; Sahai, 2009), and is not likely to be of concern in M. pinnata. Pollen storage for breeding purposes was possible at freezing temperatures and it was well maintained at -20oC and -80oC.
Facilities for -20oC are more readily available and given there was no difference in viability between -20oC and -80oC after a year, -20oC is recommended. Storage at 4oC is possible for short periods of less than three months as it retains viability at about 60%. Pollen storage at the -20oC is recommended here as it would preserve pollen within and between flowering seasons or in transit to one or more distant regions. Long term storage of viable pollen is important to preserve resources that can be utilized for breeding programs and genetic engineering (Alba et al., 2011).
Pod and seed yield were low and highly variable. These parameters improved with the introduction of honey bees but remained problematic. Pod and seed yields were about 17-fold higher with, than without honey bees. Reliance on local pollinators was not a viable option for high yields in Kununurra. The provision of additional honey bees was required to increase pollination and seed set. Pollination is obviously a critical factor for seed or fruit set in most plants, especially for commercial production of seed from M. pinnata (Dhillon et al., 2009; Raju and Rao, 2006).
Only a third of M. pinnata trees produced seeds under irrigated plantation conditions indicating inadequate pollination in Kununurra. Similarly in India, observations indicate a large proportion of trees did not produce any seeds in natural populations, however lack of quantitative
data for seed number and tree age makes comparisons difficult (Sharma et al., 2011). Presumably insects are present in natural forests but there may be competitions or other factors influencing their activity on M. pinnata.
CONCLUSION
Flowering time of M. pinnata in northern Western Australia occurred at the end of the hot, dry season, from early October to early November. Two local bees, namely Megachile sp. and Nomia sp. were identified as legitimate pollinators visited flowers. The low foraging activities of the local bees may have been due to high temperatures and low nectar availability. Introduced honey bees, Apis mellifera, was legitimate pollinators, and they substantially increased pollination and seed yield and are recommended for commercial production systems. Pollen viability was high and it could be stored short term at 4°C and long term at -20°C. Seed yield was variable and low. Further research is required to increased seed yield.
ACKNOWLEDGMENTS
We would like to thank the following organizations and people: Department of Higher Education, Republic of Indonesia (for the PhD scholarship for Ni Luh Arpiwi), Forest Products Commission of Western Australia, and the School of Plant Biology at the University of Western Australia for funding the research. Thanks also to Dr Terry Houston (Curator – Entomology, Western Australian Museum, Perth) for insect identification, Kevin and Marlene for lending beehives containing Apis meliffera and assisting with hive placement, Dr Robert Manning from DAFWA for providing pollen traps and discussions on bee pollination, and John Streatfield and Len Norris of the Forest Products Commission for their assistance in the field and with technical problems.
REFERENCES
Alba, V., Bisignano, V., Alba, E., De Stradis, A., Polignano, G.B. 2011.
Effects of cryopreservation on germinability of olive (Olea europaea L.) pollen. Genet Resour Crop Evol 58:977–982.
Arathi, H.S., Ganeshaiah, K.N., Shaanker, R.U., Hedge, S.G. 1999. Seed abortion in Pongamia pinnata (Fabaceae). Am J Bot 86: 659-662.
Aronne, G., Giovanetti, M., De Micco, V. 2012. Morphofunctional traits and pollination mechanisms of Coronilla emerus L. flowers (Fabaceae). The Scientific World Journal. doi:10.1100/2012/381575
Arpiwi, N.L., Yan, G., Barbour, E.L., Plummer, J.A. 2012. Genetic diversity, seed traits, and salinity tolerance of Millettia pin-nata (L.) Panigrahi, a biodiesel tree. Genet Resour Crop Evol. 60:677–692.
Astarini, I.A., Yan, G., Plummer, J.A. 1999. Interspecific hybridisation of Boronias. Aust J Bot 47: 851-864
Austin, P.T., Hewett, E.W., Noiton, D.A., Plummer, J.A. 1996. Cross pollination of ‘Sundrop’ apricot (Prunus armeniaca L.) by honeybees. New Zeal J Crop Hort Sci 24:287-294.
Austin, P. T., Hewett, E. W., Noiton, D., Plummer, J.A. 1998. Adjusting growth stage values to develop a linear scale for apricot flower bud phenology. HortScience 33:1141-1144.
Bhattacharya, A. 2004. Flower visitors and fruit set of Anacardium occidentale. Ann Bot Fennici 41: 385-392.
Bureau of Meteorology (2009). Climate data online http://www. bom.gov.au/climate/data/. Accessed on 20 June 2012
Dafni, A., Maues, M.M. 1998. A rapid and simple procedure to determine stigma receptivity. Sex Plant Reprod 11:177-180
Dhillon, R.S., Hooda, M.S., Ahlawat, K.S., Kumari, S. 2009. Floral biology and breeding behaviour in karanj (Pongamia pinnata L. Pierre). Indian Forester 135: 618-628.
George, N., Byrne, M., Yan, G. 2009. Observation of reproductive biology of Acacia saligna (Labill.) H.L. Wendl. Journal of Royal Society of Western Australia 92: 5-14.
Herrera, C.M. 1990. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resources availability, in a summer flowering Mediterranean shrub. Oikos 58: 277-288.
Houston, T. 2011. Native bees. Information sheet. Western Australian Museum
Kakani, V.G., Redy, K.R., Koti, S., Wallace, T.P., Prasad, P.V.V., Reddy, V.R., Zhao, D. 2005. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann Bot 96: 59–67.
Morton, J.F. 1990. The pongam tree, unfit for Florida landscaping, has multiple practical uses in under-developed lands. Proc Fla State Hort Soc 103, 338-343.
Murphy, H.T., O’Connell, D.A., Seaton, G., Raison, R.J., Rodriguez, L.C., Braid, A.L., Kriticos, D.J., Jovanovic, T., Abadi, A., Betar, M., Brodie, H., Lamont, M., McKay, M., Muirhead, G., Plummer, J., Arpiwi, N.L., Ruddle, B., Saxena, S., Scott, P.T., Stucley, C., Thistlethwaite, B., Wheaton, B., Wylie, P., Gresshoff, P.M. 2012. A common view of the opportunities, challenges and research actions for Pongamia in Australia. Bioenerg Res 5:778–800.
Sahai, K. 2009. Reproductive biology of two species of Canavalia DC. (Fabaceae)—A non-conventional wild legume Flora 204: 762–768.
Scott, P.T., Pregelj, L., Chen, N., Hadler, J.S., Djordjevic, M.A., Gresshoff, P.M. 2008. Pongamia pinnata: an untapped resource for the biofuels industry of the future. Bioenerg Res 1:2-11.
Sharma, S.S., Negi, M.S., Sinha, P., Kumar, K., Tripathi, S.B. 2011. Assessment of genetic diversity of biodiesel species Pongamia pinnata accessions using AFLP and three endonuclease-AFLP. Plant Mol Biol Rep 29:12-18.
Sujatha, K., Panda, B.M., Hazra ,S. 2008. De novo organogenesis and plant regeneration in Pongamia pinnata, oil producing tree legume. Trees 22: 711-716.
Westerkamp, C., Gottsberger, G. 2000. Review and interpretation. Diversity pays in crop pollination. Crop Science 40: 1209-1222.
23
Discussion and feedback